Fasting Part 1 gave some background information about what happens during fasting and how to prepare for a fast. So which fasting method should you choose? We dive deep into the research below and give you the specific methods appropriate for goals including fat loss, longevity, and others.
Below you can find:
- Fasting Goals – What Does the Research Say?
- Enhancing Longevity and Healthspan
- Making New Stem Cells
- Enhancing Autophagy
- Treating Cancer
- Summary: The Takeaways
This is Part 2 of the Ketosource series on fasting.
If you haven’t read it yet, Fasting Part 1 gives some background information on fasting. Part 1 describes what happens during fasting and how to prepare for a fast. Take a look at Part 1 to get some foundation before continuing with Fasting Part 2 here.
Below you will learn which fasting method is best for each fasting goal.
Check out the Summary Takeaways section at the end to see how fasting fits your specific goals. Then come back and grab all the details and references to go over with your doctor. That way you’ll be fully prepared and get the most from your fast.
Fasting Goals – What Does the Research Say?
As you’ll see below, guidelines for some of the major fasting goals are clearly defined by research on humans.
But this is not true for all the fasting goals. Some of these goals have only been demonstrated in animal models so far.
For some of these goals, it is still possible to create reasonable guidelines based on animal models. As discussed above, markers of the Fasted State help to compare the fasting timelines for humans and other animals.
Some of the major fasting goals are explained below. For each goal, the relevant fasting methods are outlined in detail. These details are also summarized in the Takeaways section at the end.
Enhancing Longevity and Healthspan
Increased longevity is often considered the Holy Grail of benefits from lifestyle interventions.
Many expect this benefit from fasting. After all, animals like nematodes and rats see longevity benefits from calorie restriction and fasting.
Nematodes and rats are separated by over 900 million years of evolution! There must be something about this type of restriction that is inherently good for longevity.
But even if fasting does increase human longevity, it would be difficult to measure. Longevity studies in humans are not practical due to the necessary time and resources.
If lifespan is the “years in your life”, then healthspan is the “life in your years.” Healthspan is a way to refer to healthy aging, i.e. how many years you can live without the burden of disease.
Markers of healthspan are much easier to measure in humans than lifespan.
Alternate-Day Fasting
Increased longevity was found in fasting rats as early as 1946. These rats fasted at least one day a week. Rats who fasted every one in three days had the best combination of healthspan benefits and increased longevity.
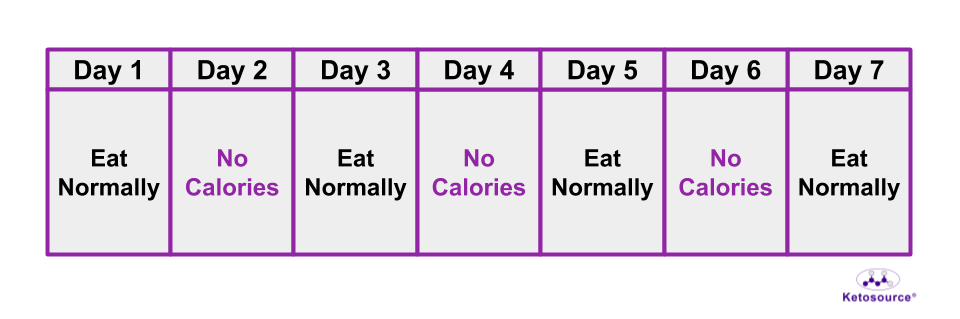
This fasting method is most similar to alternate-day fasting in humans. You can see an illustration of alternate-day fasting in Figure 10 below.
The 1946 study on fasting rats looked at breast tumors as a marker of healthspan. These tumors were assessed in female rats in the control and fasting groups. The female rats who fasted every one in three days developed smaller tumors than the control group. These tumors also appeared later in life than tumors in the control group.
Figure 10. One week of the alternate-day fasting method.
The Fasting Mimicking Diet (FMD) and Prolonged Fasting
The Fasting Mimicking Diet (FMD) improves the healthspan of mice. Like the rat study above, this includes a decrease in the number of mice with tumors. It’s important to note here that the FMD for mice is different than the FMD for humans.
(NOTE: Fasting Mimicking Diet, FMD, and Prolon are registered trademarks of L-Nutra)
For mice, this version of the FMD is four days long and begins with ~50% of normal calories on Day One. Then the mice eat ~10% of normal calories on Days 2-4.
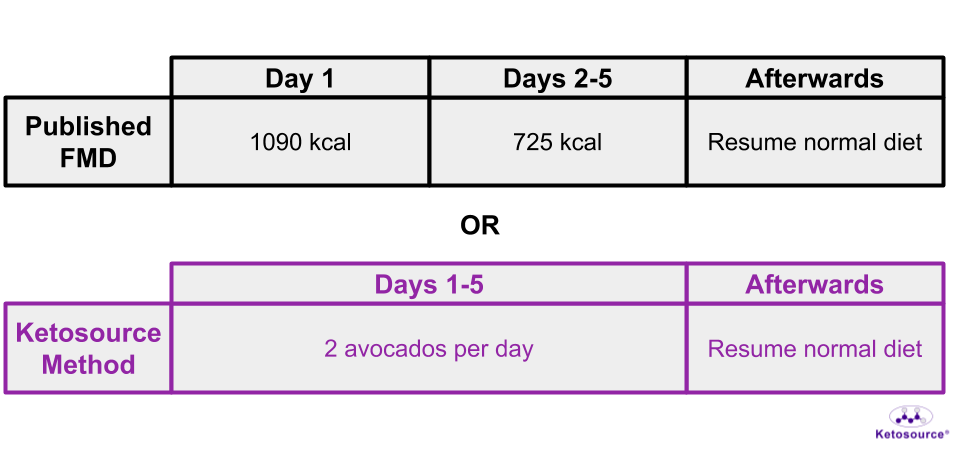
For humans, this version of the FMD is five days long with ~1090 calories on Day One and ~725 calories on Days 2-5.
If you follow a 2000 calorie/day diet, this means you’d eat ~53% of your normal calories on Day One and ~36% of your normal calories on Days 2-5.
You can see that the mouse FMD has a deeper Fasted State than the human FMD based on the % drop in calories.
As you saw in the fasting timeline graphs, ketone bodies serve as a marker of the Fasted State. Levels of ketone bodies increase ~10-fold in mice at the end of their FMD cycle. In humans, this increase is only 3.7-fold.
To see ketone body changes like the mice, you would need to fast for at least two days.
IGF1 serves as marker of the Fed State and goes down while fasting. IGF1 levels decrease by ~45% in mice by the end of their FMD cycle. In humans, IGF1 levels decrease by only 24%.
To get IGF1 levels to drop by 45% like the mice, you would need to fast for at least 2-3 days. There is a large variation in how people’s IGF1 levels drop during a fast though, so this may take as long as 5 days.
Another important difference in the mouse and human FMDs is how often they are performed. In this study, mice repeated the FMD every two weeks once they hit middle age. The humans in this study followed the FMD once a month for only three months.
This is not to say the 5-day FMD is useless for humans. In humans, three months of the 5-day FMD improves several biomarkers which can predict disease. If you have biomarkers trending in the wrong direction, the FMD can be useful to get you back on track.
But to get some of the same healthspan benefits that mice get from the FMD, three days of fasting would be a good place to start.
Using a 3-day fast once per month for three consecutive months may be even more beneficial than the current 5-day FMD method. This is based on the changes in blood glucose, ketones, and IGF1 levels. But there is no study that has tested this yet.
Alternatively, you could also modify the FMD to focus your calories on high-fat and high-fiber foods. The human 5-day FMD method includes carbohydrates and protein which take you out of the Fasted State.
An avocado-based FMD is described on The Quantified Body podcast which you can find here. This will be referred to as the Ketosource Method from now on. Some results from one cycle of the Ketosource Method show an even deeper Fasted State than the mouse FMD. You can see a breakdown of the published FMD and the Ketource Method in Figure 11 below.
Interestingly, IGF1 levels dropped by 44% in the Ketosource Method. This suggests that one cycle of the Ketosource Method is at least equivalent to one cycle of the mouse FMD.
To get even more benefits, you could do two or more cycles of the Ketosource Method every month.
Figure 11. Different approaches to the 5-day FMD.
Time-Restricted Feeding (TRF)
The FMD was developed to mimic prolonged fasting. But it is not the first diet designed to do this. Over a century ago, the ketogenic diet was developed as a fasting replacement for the treatment of epilepsy.
Recent mouse studies on the ketogenic diet showed improved mid-life mortality and median lifespan. One key difference between these two studies is how they dealt with food intake.
Roberts, et al. controlled the calorie intake of mice, which means the researchers likely fed the mice at the same time every day. In this way, the median lifespan increase for these mice could have come from TRF.
In support of this, mice eating one meal a day have increased longevity and healthspan. This seems to be independent of what they eat or how much they eat.
As mentioned already, the fasting timelines for mice and humans are very different. There is no great way to compare these benefits for mice with potential TRF benefits for humans.
But there are some modest benefits for people following TRF.
For example, most people eat within a 15-hour window that extends into the night. Eating between 6 AM and 7 PM could prevent overeating and lead to weight loss.
An 11-hour eating window (13-hour overnight fast) is also associated with less breast cancer recurrence.
An 8-hour eating window shows some benefit for obese people and even for athletes.
Those with prediabetes or at risk for type 2 diabetes can see benefits from eating within 6 hours and 9 hours, respectively. In these cases, the eating window should begin in the morning (8-9 AM).
Making New Stem Cells
One of the most overlooked benefits of fasting is its effect on stem cells.
When you get an injury, stem cells replace the dead cells and regenerate the damaged area. But it is well known that this ability of stem cells decreases with age.
Hematopoietic stem cells, or stem cells of the blood system, illustrate this well.
The number of hematopoietic stem cells in mice increases as they age. But hematopoietic stem cells from aged mice are more defective than the same cells from young mice.
Prolonged Fasting
Water-only fasting for three days in mice improves the function of their hematopoietic stem cells.
This length of fasting causes the mice to create more stem cells. But unlike aging, in this case the new stem cells are more functional than the old stem cells.
This was evident from the ratio of blood cell types which looked more like young mice after fasting. In this way, fasting “rejuvenated” the blood systems of these mice.
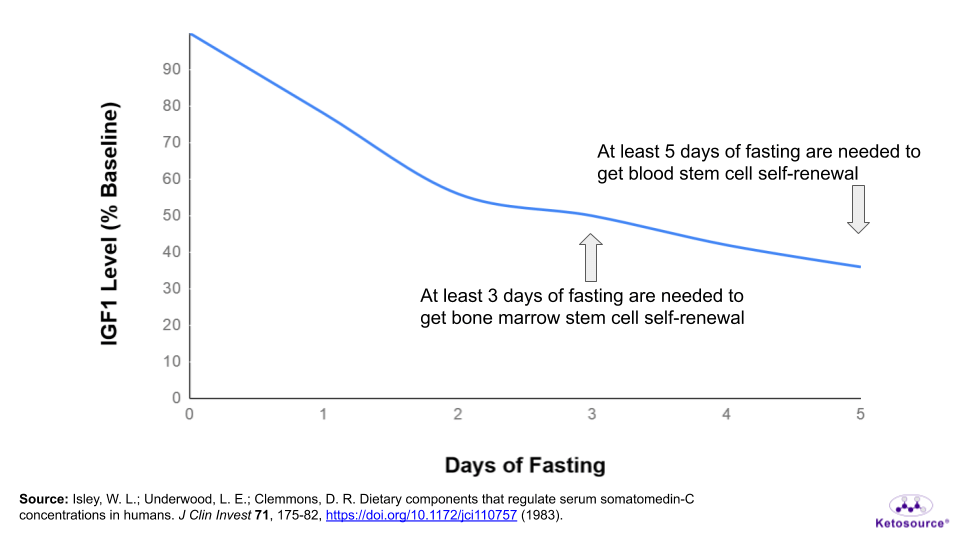
These benefits are believed to come from a drop in IGF1 levels in the mice. At the end of a 3-day fast, IGF1 levels in mice drop by 70%.
You would need to fast for at least 5 days to get a similar drop in IGF1 levels. As stated earlier, people’s IGF1 levels drop at different rates during fasting. It could take as long as 9 days of fasting to see this drop in IGF1.
The Fasting Mimicking Diet (FMD)
The FMD also provides stem cell benefits to mice. This includes benefits to brain stem cells and stem cells found in the bone marrow.
Interestingly, certain organs shrink (kidney, heart, liver) in mice while they follow the FMD. These organs then return to their normal weights or heavier after the mice eat again for a week. There are also signs of organ and blood system rejuvenation following this re-feeding phase.
This highlights the importance of the re-feeding phase. Without entering back into this Fed State after the Fasted State, the mice would not be able to use their benefits from their Fasted State.
As discussed in the “Enhancing Longevity and Healthspan” section, the mouse FMD has a deeper Fasted State than the human 5-day FMD. But you can mimic the mouse FMD with water-only fasting for 3-5 days or by using the Ketosource Method as an FMD.
Figure 12. Where stem cell benefits fall on the fasting timeline.
Enhancing Autophagy
Autophagy is the process whereby old or damaged cellular machinery gets recycled.
Autophagy is integral to many cellular functions, and a background level of autophagy is found in practically all cells. This means autophagy benefits come from ways to “enhance” autophagy, which already takes place to some degree.
Certain natural compounds which promote longevity in multiple species do so via autophagy. Mouse studies have also found that coffee and exercise enhance autophagy. Unfortunately, no studies have confirmed these findings in humans yet.
However, water-only fasting can enhance autophagy in both mice and humans.
Research suggests the timelines are different for each species. And that the timelines are also unique for different cells in the body.
Autophagy is difficult to address as a fasting goal. One reason for this is how complex autophagy is to measure.
As explained below, insulin levels are the best way to compare the autophagy timelines for mice and humans right now. There is a lot of interest in tracking autophagy in humans, so insulin levels could be one way to track this.
Prolonged Fasting
In mice, autophagy is enhanced in muscle cells after one day of fasting, and this is shut off by the hormone insulin. This suggests that insulin levels can be used to compare the animal and human timelines for autophagy.
After one day of fasting, levels of insulin in mice have dropped by 95%.
You would need to fast for at least 2 days to get this drop in insulin. However, older human studies suggest that a 95% drop in insulin does not happen during fasting. Of course, the starting point for insulin could also matter here.
For mice, this 95% drop in insulin could be a plateau. This means it could be the lowest insulin level possible for mice. In this case, the human equivalent would likely be 5 days of fasting.
In support of this, there are mixed signals for autophagy in human muscle cells after 3 days of fasting.
This could be because insulin has not dropped enough yet to enhance autophagy. Or it could be due to the difficulty in measuring autophagy and interpreting data for the protein p62.
Autophagy usually degrades p62. The muscle cells showed some signs of autophagy in this study, but they also had higher levels of p62.
A study on white blood cells found that autophagy might be enhanced in many types of human white blood cells within 4 days of fasting. But detailed testing was only carried out after one day of fasting. This detailed testing revealed that autophagy is enhanced in just one type of white blood cell (the neutrophil) in humans after one day of fasting.
In mice, autophagy is enhanced in all types of white blood cells within two days of fasting. Could these autophagy benefits have started earlier in the fast? The researchers did not check. So for now, there is no great way to compare autophagy timelines yet for white blood cells.
Taken together, insulin is the only trackable biomarker to use when comparing the human and mouse autophagy timelines.
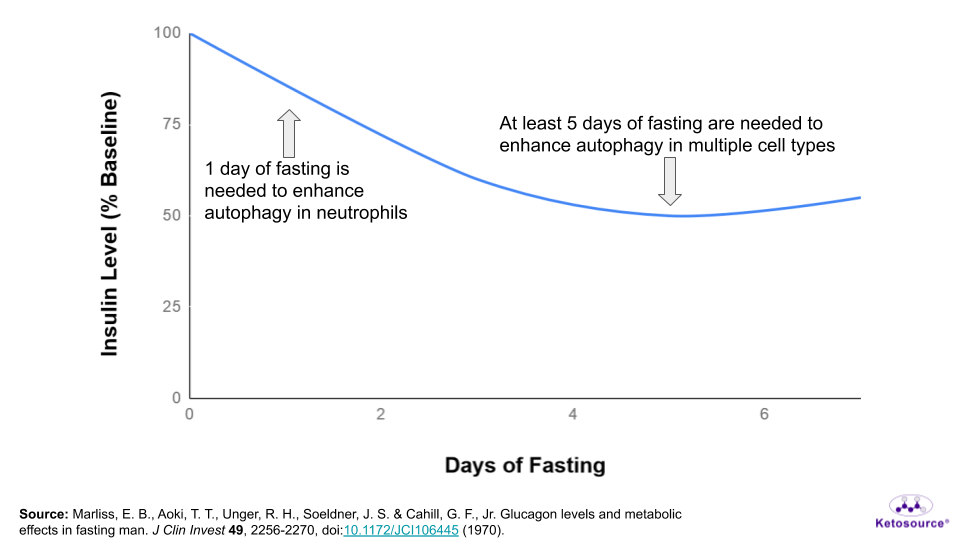
So for now, these studies suggest that you would need to fast for at least one day to enhance autophagy in one cell type (the neutrophil).
But based on the muscle studies and insulin levels during fasting, most likely at least five days of fasting are necessary to get autophagy benefits in multiple cell types. This is shown in the graph below.
Figure 13. Where autophagy benefits fall on the fasting timeline.
The Fasting Mimicking Diet (FMD)
There are signs of muscle autophagy benefits in mice following the FMD.
Mice following the FMD have decreased expression of the protein p62 in muscle. As stated earlier, p62 is degraded by autophagy.
However, this result was also seen in mice who had followed cycles of the FMD consistently for a long time. And the level of p62 in these mice on the FMD was similar to that of young mice not on the FMD.
This suggests that mice who regularly follow the FMD are more able to undergo muscle autophagy than normal mice of the same age.
Based on the role of p62 in tumor formation, this might also help explain why these mice had fewer tumors when they died.
Autophagy markers were not measured in humans in the study. And like the mouse study on muscle autophagy, the insulin levels for mice drop by ~90% during the FMD.
As stated earlier, this level of insulin is seen in humans after 5 days of fasting. This suggests that regularly fasting for 5 days could enhance your long-term autophagy benefits. In other words, it could ensure you maintain the ability to undergo autophagy as you age.
Treating Cancer
This Is Not a Recommendation
The use of fasting in cancer treatment is very controversial. This section is not intended to promote the use of fasting as an adjuvant to, or as a replacement for cancer treatment.
Nor is it intended to offer guidelines for treating cancer. It is intended only to serve as reference material which you can go over with your physician.
As mentioned above, the Fasted State comes with lower levels of the pro-growth hormones IGF1 and insulin. The Fasted State also comes with lower blood glucose levels and higher blood ketone body levels.
Insulin and IGF1 are both implicated in the development of cancer and in its progression. Inhibiting the pro-growth effects of these hormones is a major focus in cancer drug development.
Similarly, glucose metabolism is another target for cancer therapy. An estimated >90% of cancers show signs of enhanced glucose metabolism.
Studies suggest that cancer arises due to problems with cellular metabolism. And that these problems make cancer cells rely on glucose.
During fasting, normal cells can deal with a shift from glucose metabolism to ketone body metabolism. In fact, nearly 60% of brain metabolism shifts to using ketone bodies during long fasts.
Glucose levels as low as 0.5 mM (9 mg/dL) have been recorded during fasting without symptoms of hypoglycemia. This is only ~10% of a normal blood glucose level. This low blood glucose level would be fatal without the high levels of ketone bodies that come from fasting.
As stated above, lowering glucose and pro-growth hormones while increasing ketone bodies is a key feature of the Fasted State. This is believed to selectively harm cancer cells.
Prolonged Fasting
Mice with 70-80% lower IGF1 levels are more protected against the side effects of chemotherapy than normal mice. These mice also have better survival against melanoma. This is the IGF1 level reached by normal mice after fasting for three days.
Mice who fast for three days are also protected against the side effects of chemotherapy. Together, these studies suggest that a ~70% drop in IGF1 levels is beneficial for chemotherapy side effects and cancer survival in mice.
As mentioned earlier, humans need to fast for at least 5 days to see this drop in IGF1 levels.
Have Cancer Patients Fasted for Five Days?
There are at least three studies on prolonged fasting in cancer patients. In each of these studies, the fasting period was right before and/or right after chemotherapy. Two of the studies were on the same group of patients.
None of the studies focused on fasting as a treatment for cancer. But each of them suggested that fasting can be safe for cancer patients undergoing chemotherapy.
In one of these studies, ten patients with various cancers fasted for 48-140 hours before and/or 5-56 hours after chemotherapy. On average, fatigue and weakness from the chemotherapy were better when the patients were fasting. None of the chemotherapy side effects became worse.
For some of the patients of this study, the effectiveness of their chemotherapy could be measured. In these cases, there were no signs that fasting prevented the effectiveness of chemotherapy against their cancers.
In the second study, 20 patients with various cancers fasted for 1-3 days surrounding chemotherapy. This fasting period allowed for up to 200 calories per day. The changes in glucose, ketones, and pro-growth hormones varied amongst the patients.
Like the mouse study mentioned above, there were benefits for white blood cells in patients who fasted for 2-3 days. Specifically, their white blood cells were protected against DNA damage from the chemotherapy.
The third study looked at the same group of patients as the second study. This third study also found that white blood cells were more protected in patients who fasted for three days compared to patients who fasted for one day.
Together, these three studies suggest that fasting can be safe for cancer patients and it can help with the side effects of chemotherapy. These studies also suggest that fasting for 2-3 days can help protect the immune system from chemotherapy side effects.
But it’s important to remember that these fasts were carried out under medical supervision. Malnutrition and muscle wasting are still some of the major concerns for the use of fasting in cancer treatment. More research is needed before a specific fasting method is proven safe and effective for cancer patients.
Summary: The Takeaways
The best fasting method for you is based on your specific goal(s), lifestyle, and any medications you currently take.
Takeaways: How to Achieve Your Fasting Goals
-
Weight Loss: Use alternate-day fasting to lose weight. This method has a high compliance rate and keeps you at a big calorie deficit for the week.
-
Enhancing Longevity and Healthspan: Use 3 monthly cycles of the Ketosource Method (found here) to get disease markers under control. Use a 5-day fast in place of the FMD to add autophagy and stem cell benefits. Repeat as necessary if your blood markers start trending in the wrong direction.
-
Enhancing Autophagy: A 1-day fast will enhance autophagy in one cell type (the neutrophil). Use a 5-day fast to enhance autophagy for multiple cell types.
-
Stem Cell Self-Renewal: Use a 5-day fast to promote stem cell self-renewal. Depending on how IGF1 levels drop for you, this could require up to nine days of fasting.
-
Treating Cancer: More research is needed to clarify the safety and efficacy of fasting in cancer patients. It is too early to set any takeaways for the role of fasting in cancer treatment.
-
Treating Type 2 Diabetes: Use modified fasting so you can take your medications safely. Use more than two low-calorie days per week to lose weight more quickly. Add an early 6-9 hour TRF on top of this fasting method to get more benefit.
References
{5748374:JE5J8BEP},{5748374:83UM7QL8};{5748374:ES9NPU2S},{5748374:MF4ZZAX3};{5748374:TYQH7SPE};{5748374:ES9NPU2S};{5748374:ES9NPU2S};{5748374:3HHU6VZN};{5748374:ES9NPU2S};{5748374:3HHU6VZN};{5748374:3HHU6VZN};{5748374:3HHU6VZN};{5748374:3HHU6VZN};{5748374:9R8B7N39};{5748374:3HHU6VZN};{5748374:P2Z6B7GC};{5748374:E6IMUMCA};{5748374:3HHU6VZN};{5748374:SQU9ZGIQ};{5748374:SQU9ZGIQ},{5748374:3HHU6VZN},{5748374:WX6XVYQC},{5748374:P2Z6B7GC};{5748374:3HHU6VZN};{5748374:3HHU6VZN},{5748374:WZLT2AEX};{5748374:WZLT2AEX};{5748374:3HHU6VZN};{5748374:3HHU6VZN};{5748374:VMKSQVPV};{5748374:9N4I95G9};{5748374:3YQ4G2FK};{5748374:3YQ4G2FK};{5748374:HZ5GBZQF};{5748374:HZ5GBZQF};{5748374:8J8FRJPG};{5748374:CXS8BFWR};{5748374:S6L3EE64};{5748374:CUMUP9TI};{5748374:XIPWH7MH};{5748374:C6HDVJ7S};{5748374:4NRKPFQR};{5748374:J7ZVR9HH};{5748374:4NB77YNV};{5748374:6NCTSX67};{5748374:XL67M6CI};{5748374:3WVQ9RWW};{5748374:XL67M6CI};{5748374:3WVQ9RWW};{5748374:3WVQ9RWW};{5748374:3WVQ9RWW};{5748374:338PM6JJ};{5748374:P2Z6B7GC};{5748374:E6IMUMCA};{5748374:3HHU6VZN};{5748374:3HHU6VZN};{5748374:3HHU6VZN},{5748374:P2Z6B7GC};{5748374:WZLT2AEX};{5748374:8FYI8Q6H};{5748374:R2BJ3B97},{5748374:CE2PQYYN};{5748374:ZGACAGRI};{5748374:L5K2CMYB};{5748374:4XGWAUUR};{5748374:P4DNECM5};{5748374:TWZWWXS5};{5748374:9R8B7N39};{5748374:TWZWWXS5};{5748374:4TLPENTP};{5748374:3XRK86DQ};{5748374:AAWHJF5X};{5748374:I2Y8YCZK};{5748374:3XRK86DQ};{5748374:XTBGIK8S};{5748374:XTBGIK8S};{5748374:XTBGIK8S};{5748374:3XRK86DQ},{5748374:P4DNECM5};{5748374:TWZWWXS5},{5748374:4TLPENTP};{5748374:3HHU6VZN};{5748374:3HHU6VZN};{5748374:I2Y8YCZK};{5748374:3HHU6VZN};{5748374:AFC6LMAY};{5748374:3HHU6VZN};{5748374:3HHU6VZN};{5748374:TWZWWXS5},{5748374:P4DNECM5};{5748374:3HHU6VZN};{5748374:4TLPENTP};{5748374:GJB9K3L2};{5748374:P2Z6B7GC};{5748374:WX6XVYQC};{5748374:WX6XVYQC};{5748374:MDJEWPB4};{5748374:UMACU7KS};{5748374:HRIQWFPR};{5748374:PWKABTDZ};{5748374:UMACU7KS};{5748374:ZA4XJNFE};{5748374:QWP452KJ};{5748374:9R8B7N39};{5748374:WX6XVYQC},{5748374:E6IMUMCA};{5748374:UMACU7KS};{5748374:338PM6JJ};{5748374:3WVQ9RWW};{5748374:E6IMUMCA},{5748374:P2Z6B7GC};{5748374:NCTGYSIU},{5748374:ZZT2RDW2},{5748374:3WVQ9RWW};{5748374:NCTGYSIU};{5748374:NCTGYSIU};{5748374:ZZT2RDW2};{5748374:3WVQ9RWW};{5748374:ZZT2RDW2};{5748374:3WVQ9RWW};{5748374:ZZT2RDW2},{5748374:3WVQ9RWW},{5748374:NCTGYSIU};{5748374:GJB9K3L2}
3d-printed-materials-and-systems
default
asc
0
8655
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A950%2C%22request_next%22%3A50%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22PNNNMDCY%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Dietze%20et%20al.%22%2C%22parsedDate%22%3A%221976%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EDietze%20G%2C%20Wicklmayr%20M%2C%20Grunst%20J%2C%20Mehnert%20H%20%281976%29%20%5BThe%20anti-ketogenic%20effect%20of%20fructose%5D.%20Verh%20Dtsch%20Ges%20Inn%20Med%2082%20Pt%201%3A767%26%23x2013%3B769%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22%5BThe%20anti-ketogenic%20effect%20of%20fructose%5D%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22G.%22%2C%22lastName%22%3A%22Dietze%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Wicklmayr%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Grunst%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H.%22%2C%22lastName%22%3A%22Mehnert%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221976%22%2C%22language%22%3A%22ger%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%220070-4067%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222020-04-17T10%3A10%3A51Z%22%7D%7D%2C%7B%22key%22%3A%22UMT4FS8S%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lee%20and%20Wolever%22%2C%22parsedDate%22%3A%221998-12%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ELee%20BM%2C%20Wolever%20TM%20%281998%29%20Effect%20of%20glucose%2C%20sucrose%20and%20fructose%20on%20plasma%20glucose%20and%20insulin%20responses%20in%20normal%20humans%3A%20comparison%20with%20white%20bread.%20Eur%20J%20Clin%20Nutr%2052%3A924%26%23x2013%3B928.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fsj.ejcn.1600666%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fsj.ejcn.1600666%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Effect%20of%20glucose%2C%20sucrose%20and%20fructose%20on%20plasma%20glucose%20and%20insulin%20responses%20in%20normal%20humans%3A%20comparison%20with%20white%20bread%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B.%20M.%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%20M.%22%2C%22lastName%22%3A%22Wolever%22%7D%5D%2C%22abstractNote%22%3A%22OBJECTIVE%3A%20To%20determine%20the%20plasma%20glucose%20and%20insulin%20responses%20of%20various%20doses%20of%20glucose%2C%20sucrose%2C%20fructose%20and%20white%20bread%20in%20normal%20human%20subjects.%5CnDESIGN%3A%20Plasma%20glucose%20and%20insulin%20were%20measured%20before%20and%20at%20various%20times%20after%208%20subjects%20ate%2013%20different%20test%20meals%20in%20randomized%20order%20on%20separate%20days%20after%20an%20overnight%20fast.%20Test%20meals%20consisted%20of%20500%20ml%20of%20tea%20or%20water%20to%20which%20was%20added%20either%20nothing%2C%2025%2C%2050%2C%20or%20100%20g%20of%20glucose%20or%20sucrose%2C%2025%20or%2050%20g%20fructose%2C%2050%20g%20glucose%20plus%2050%20g%20fructose%2C%20or%20a%2025%2C%2050%20or%20100%20g%20carbohydrate%20portion%20of%20white%20bread.%20The%20glycaemic%20%28GI%29%20and%20insulinaemic%20index%20%28II%29%20values%20of%20the%20sugars%20were%20calculated%20by%20expressing%20the%20incremental%20areas%20under%20the%20plasma%20glucose%20and%20insulin%20curves%20%28AUC%29%20after%20glucose%2C%20sucrose%20and%20fructose%20as%20a%20percentage%20of%20the%20respective%20AUC%20after%20white%20bread%20containing%20the%20same%20amount%20of%20carbohydrate.%5CnSETTING%3A%20University%20teaching%20hospital%20clinical%20nutrition%20centre.%5CnSUBJECTS%3A%20Lean%2C%20normal%20subjects%20%284%20male%2C%204%20female%29%2021-33%20y%20of%20age.%5CnRESULTS%3A%20Plasma%20insulin%20responses%20increased%20nearly%20linearly%20as%20carbohydrate%20intake%20increased%20from%200%20to%20100%20g%2C%20but%20glycaemic%20responses%20increased%20by%20only%2068%25%20and%2038%25%20as%20carbohydrate%20intake%20increased%20from%2025%20to%2050%20g%20and%2050%20to%20100g%2C%20respectively.%20The%20GI%20and%20II%20values%20of%20glucose%2C%20149%2B%5C%2F-16%20and%20147%2B%5C%2F-18%2C%20respectively%2C%20were%20significantly%20greater%20than%20those%20of%20bread%20%28100%3B%20P%3C0.05%29%2C%20while%20the%20values%20for%20fructose%2C%2016%2B%5C%2F-4%20and%2022%2B%5C%2F-3%20were%20significantly%20less%20than%20those%20of%20bread%20%28P%3C0.001%29.%20GI%20values%20did%20not%20differ%20significantly%20from%20II%20values.%5CnCONCLUSIONS%3A%20It%20is%20concluded%20that%2C%20in%20normal%20subjects%2C%20as%20carbohydrate%20intake%20is%20increased%20from%200%20to%20100%20g%2C%20plasma%20insulin%20responses%20increase%20at%20a%20greater%20rate%20than%20plasma%20glucose%20responses.%20The%20insulinaemic%20responses%20elicited%20by%20glucose%2C%20sucrose%20or%20fructose%20are%20similar%20to%20those%20that%20would%20be%20expected%20from%20a%20starchy%20food%20with%20the%20same%20glycaemic%20index.%22%2C%22date%22%3A%22Dec%201998%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1038%5C%2Fsj.ejcn.1600666%22%2C%22ISSN%22%3A%220954-3007%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%226ZD5MYAL%22%5D%2C%22dateModified%22%3A%222020-04-17T10%3A08%3A36Z%22%7D%7D%2C%7B%22key%22%3A%226X22CVSE%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EHypometabolism%20as%20a%20therapeutic%20target%20in%20Alzheimer%26%23x2019%3Bs%20disease%20%7C%20SpringerLink.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Flink.springer.com%5C%2Farticle%5C%2F10.1186%5C%2F1471-2202-9-S2-S16%27%3Ehttps%3A%5C%2F%5C%2Flink.springer.com%5C%2Farticle%5C%2F10.1186%5C%2F1471-2202-9-S2-S16%3C%5C%2Fa%3E.%20Accessed%2030%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Hypometabolism%20as%20a%20therapeutic%20target%20in%20Alzheimer%27s%20disease%20%7C%20SpringerLink%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flink.springer.com%5C%2Farticle%5C%2F10.1186%5C%2F1471-2202-9-S2-S16%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T11%3A48%3A44Z%22%7D%7D%2C%7B%22key%22%3A%22VCPKRBSX%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EDietary%20ketosis%20enhances%20memory%20in%20mild%20cognitive%20impairment%20-%20ScienceDirect.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fabs%5C%2Fpii%5C%2FS0197458010004392%27%3Ehttps%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fabs%5C%2Fpii%5C%2FS0197458010004392%3C%5C%2Fa%3E.%20Accessed%2030%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Dietary%20ketosis%20enhances%20memory%20in%20mild%20cognitive%20impairment%20-%20ScienceDirect%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fabs%5C%2Fpii%5C%2FS0197458010004392%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T11%3A46%3A44Z%22%7D%7D%2C%7B%22key%22%3A%22BDH7M7EZ%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EAlzheimer%26%23x2019%3Bs%20Disease%20is%20Type%203%20Diabetes%26%23x2014%3BEvidence%20Reviewed%20-%20Suzanne%20M.%20de%20la%20Monte%2C%20Jack%20R.%20Wands%2C%202008.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fjournals.sagepub.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1177%5C%2F193229680800200619%27%3Ehttps%3A%5C%2F%5C%2Fjournals.sagepub.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1177%5C%2F193229680800200619%3C%5C%2Fa%3E.%20Accessed%2030%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Alzheimer%27s%20Disease%20is%20Type%203%20Diabetes%5Cu2014Evidence%20Reviewed%20-%20Suzanne%20M.%20de%20la%20Monte%2C%20Jack%20R.%20Wands%2C%202008%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fjournals.sagepub.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1177%5C%2F193229680800200619%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T11%3A42%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22RWXZB9WD%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EFeasibility%20and%20efficacy%20data%20from%20a%20ketogenic%20diet%20intervention%20in%20Alzheimer%26%23x2019%3Bs%20disease%20-%20ScienceDirect.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS2352873717300707%27%3Ehttps%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS2352873717300707%3C%5C%2Fa%3E.%20Accessed%2030%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Feasibility%20and%20efficacy%20data%20from%20a%20ketogenic%20diet%20intervention%20in%20Alzheimer%27s%20disease%20-%20ScienceDirect%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS2352873717300707%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T11%3A27%3A13Z%22%7D%7D%2C%7B%22key%22%3A%22E7CGIUQT%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Pawlosky%20et%20al.%22%2C%22parsedDate%22%3A%222020-02-04%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EPawlosky%20RJ%2C%20Kashiwaya%20Y%2C%20King%20MT%2C%20Veech%20RL%20%282020%29%20A%20Dietary%20Ketone%20Ester%20Normalizes%20Abnormal%20Behavior%20in%20a%20Mouse%20Model%20of%20Alzheimer%26%23x2019%3Bs%20Disease.%20IJMS%2021%3A1044.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijms21031044%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijms21031044%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20Dietary%20Ketone%20Ester%20Normalizes%20Abnormal%20Behavior%20in%20a%20Mouse%20Model%20of%20Alzheimer%5Cu2019s%20Disease%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Robert%20J.%22%2C%22lastName%22%3A%22Pawlosky%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yoshihero%22%2C%22lastName%22%3A%22Kashiwaya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%20Todd%22%2C%22lastName%22%3A%22King%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%20L.%22%2C%22lastName%22%3A%22Veech%22%7D%5D%2C%22abstractNote%22%3A%22Because%20of%20a%20decreased%20sensitivity%20toward%20insulin%2C%20a%20key%20regulator%20of%20pyruvate%20dehydrogenase%20%28PDH%29%2C%20Alzheimer%5Cu2019s%20patients%20have%20lower%20brain%20glucose%20utilization%20with%20reductions%20in%20Tricarboxylic%20Acid%20%28TCA%29%20cycle%20metabolites%20such%20as%20citrate%2C%20a%20precursor%20to%20n-acetyl-aspartate.%20In%20the%203xTgAd%20mouse%20model%20of%20Alzheimer%5Cu2019s%20disease%20%28AD%29%2C%20aging%20mice%20also%20demonstrate%20low%20brain%20glucose%20metabolism.%20Ketone%20metabolism%20can%20overcome%20PDH%20inhibition%20and%20restore%20TCA%20cycle%20metabolites%2C%20thereby%20enhancing%20amino%20acid%20biosynthesis.%20A%20ketone%20ester%20of%20d-%5Cu03b2-hydroxybutyrate%20was%20incorporated%20into%20a%20diet%20%28Ket%29%20and%20fed%20to%203xTgAd%20mice.%20A%20control%20group%20was%20fed%20a%20calorically%20matched%20diet%20%28Cho%29.%20At%2015%20months%20of%20age%2C%20the%20exploratory%20and%20avoidance-related%20behavior%20patterns%20of%20the%20mice%20were%20evaluated.%20At%2016.5%20months%20of%20age%2C%20the%20animals%20were%20euthanized%2C%20and%20their%20hippocampi%20were%20analyzed%20for%20citrate%2C%20%5Cu03b1-ketoglutarate%2C%20and%20amino%20acids.%20In%20the%20hippocampi%20of%20the%20Ket-fed%20mice%2C%20there%20were%20higher%20concentrations%20of%20citrate%20and%20%5Cu03b1-ketoglutarate%20as%20well%20as%20higher%20concentrations%20of%20glutamate%2C%20aspartate%20and%20n-acetyl-aspartate%20compared%20with%20controls.%20There%20were%20positive%20associations%20between%20%281%29%20concentrations%20of%20aspartate%20and%20n-acetyl-aspartate%20%28n%20%3D%2014%2C%20R%20%3D%200.9327%29%2C%20and%20%282%29%20%5Cu03b1-ketoglutarate%20and%20glutamate%20%28n%20%3D%2014%2C%20R%20%3D%200.8521%29%20in%20animals%20maintained%20on%20either%20diet.%20Hippocampal%20n-acetyl-aspartate%20predicted%20the%20outcome%20of%20several%20exploratory%20and%20avoidance-related%20behaviors.%20Ketosis%20restored%20citrate%20and%20%5Cu03b1-ketoglutarate%20in%20the%20hippocampi%20of%20aging%20mice.%20Higher%20concentrations%20of%20n-acetyl-aspartate%20corresponded%20with%20greater%20exploratory%20activity%20and%20reduced%20avoidance-related%20behavior.%22%2C%22date%22%3A%222020-02-04%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fijms21031044%22%2C%22ISSN%22%3A%221422-0067%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F1422-0067%5C%2F21%5C%2F3%5C%2F1044%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T11%3A22%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22N7L4Y9YZ%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rafi%20and%20Alavi%22%2C%22parsedDate%22%3A%222017-08-30%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ERafi%20MA%2C%20Alavi%20A%20%282017%29%20Debate%20on%20human%20aging%20and%20lifespan.%20Bioimpacts%207%3A135%26%23x2013%3B137.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.15171%5C%2Fbi.2017.16%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.15171%5C%2Fbi.2017.16%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Debate%20on%20human%20aging%20and%20lifespan%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mohammad%20A.%22%2C%22lastName%22%3A%22Rafi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Abass%22%2C%22lastName%22%3A%22Alavi%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222017-08-30%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.15171%5C%2Fbi.2017.16%22%2C%22ISSN%22%3A%222228-5660%2C%202228-5652%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fbi.tbzmed.ac.ir%5C%2FAbstract%5C%2Fbi-17493%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A48%3A50Z%22%7D%7D%2C%7B%22key%22%3A%22EX3J7PVP%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Crimmins%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ECrimmins%20EM%20%282015%29%20Lifespan%20and%20Healthspan%3A%20Past%2C%20Present%2C%20and%20Promise.%20GERONT%2055%3A901%26%23x2013%3B911.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fgeront%5C%2Fgnv130%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fgeront%5C%2Fgnv130%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Lifespan%20and%20Healthspan%3A%20Past%2C%20Present%2C%20and%20Promise%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eileen%20M.%22%2C%22lastName%22%3A%22Crimmins%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2212%5C%2F2015%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1093%5C%2Fgeront%5C%2Fgnv130%22%2C%22ISSN%22%3A%220016-9013%2C%201758-5341%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Facademic.oup.com%5C%2Fgerontologist%5C%2Farticle-lookup%5C%2Fdoi%5C%2F10.1093%5C%2Fgeront%5C%2Fgnv130%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A48%3A09Z%22%7D%7D%2C%7B%22key%22%3A%223JWL79XX%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Huang%20and%20Mark%20Jacquez%22%2C%22parsedDate%22%3A%222017-05-28%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EHuang%20Y%2C%20Mark%20Jacquez%20G%20%282017%29%20Identification%20of%20a%20Blue%20Zone%20in%20a%20Typical%20Chinese%20Longevity%20Region.%20IJERPH%2014%3A571.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijerph14060571%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijerph14060571%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Identification%20of%20a%20Blue%20Zone%20in%20a%20Typical%20Chinese%20Longevity%20Region%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yi%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Geoffrey%22%2C%22lastName%22%3A%22Mark%20Jacquez%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222017-05-28%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fijerph14060571%22%2C%22ISSN%22%3A%221660-4601%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F1660-4601%5C%2F14%5C%2F6%5C%2F571%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A44%3A11Z%22%7D%7D%2C%7B%22key%22%3A%22BBWNJ6WM%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Poulain%20et%20al.%22%2C%22parsedDate%22%3A%222004%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EPoulain%20M%2C%20Pes%20GM%2C%20Grasland%20C%2C%20et%20al%20%282004%29%20Identification%20of%20a%20geographic%20area%20characterized%20by%20extreme%20longevity%20in%20the%20Sardinia%20island%3A%20the%20AKEA%20study.%20Experimental%20Gerontology%2039%3A1423%26%23x2013%3B1429.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.exger.2004.06.016%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.exger.2004.06.016%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Identification%20of%20a%20geographic%20area%20characterized%20by%20extreme%20longevity%20in%20the%20Sardinia%20island%3A%20the%20AKEA%20study%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%22%2C%22lastName%22%3A%22Poulain%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Giovanni%20Mario%22%2C%22lastName%22%3A%22Pes%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Claude%22%2C%22lastName%22%3A%22Grasland%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ciriaco%22%2C%22lastName%22%3A%22Carru%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Luigi%22%2C%22lastName%22%3A%22Ferrucci%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Giovannella%22%2C%22lastName%22%3A%22Baggio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Claudio%22%2C%22lastName%22%3A%22Franceschi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Luca%22%2C%22lastName%22%3A%22Deiana%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%229%5C%2F2004%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.exger.2004.06.016%22%2C%22ISSN%22%3A%2205315565%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS0531556504002141%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A42%3A41Z%22%7D%7D%2C%7B%22key%22%3A%22BWIHNYER%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EMetabolic%20Syndrome%20and%20Risk%20of%20Cancer%20%7C%20Diabetes%20Care.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fcare.diabetesjournals.org%5C%2Fcontent%5C%2F35%5C%2F11%5C%2F2402.abstract%27%3Ehttps%3A%5C%2F%5C%2Fcare.diabetesjournals.org%5C%2Fcontent%5C%2F35%5C%2F11%5C%2F2402.abstract%3C%5C%2Fa%3E.%20Accessed%2030%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Metabolic%20Syndrome%20and%20Risk%20of%20Cancer%20%7C%20Diabetes%20Care%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fcare.diabetesjournals.org%5C%2Fcontent%5C%2F35%5C%2F11%5C%2F2402.abstract%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A26%3A51Z%22%7D%7D%2C%7B%22key%22%3A%22HIHTSGMM%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Esposito%20et%20al.%22%2C%22parsedDate%22%3A%222012-11-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EEsposito%20K%2C%20Chiodini%20P%2C%20Colao%20A%2C%20et%20al%20%282012%29%20Metabolic%20Syndrome%20and%20Risk%20of%20Cancer%3A%20A%20systematic%20review%20and%20meta-analysis.%20Diabetes%20Care%2035%3A2402%26%23x2013%3B2411.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.2337%5C%2Fdc12-0336%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.2337%5C%2Fdc12-0336%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Metabolic%20Syndrome%20and%20Risk%20of%20Cancer%3A%20A%20systematic%20review%20and%20meta-analysis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%22%2C%22lastName%22%3A%22Esposito%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Chiodini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Colao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Lenzi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22D.%22%2C%22lastName%22%3A%22Giugliano%22%7D%5D%2C%22abstractNote%22%3A%22OBJECTIVEdAvailable%20evidence%20supports%20the%20emerging%20hypothesis%20that%20metabolic%20syndrome%20may%20be%20associated%20with%20the%20risk%20of%20some%20common%20cancers.%20We%20did%20a%20systematic%20review%20and%20metaanalysis%20to%20assess%20the%20association%20between%20metabolic%20syndrome%20and%20risk%20of%20cancer%20at%20different%20sites.%20RESEARCH%20DESIGN%20AND%20METHODSdWe%20conducted%20an%20electronic%20search%20for%20articles%20published%20through%20October%202011%20without%20restrictions%20and%20by%20reviewing%20reference%20lists%20from%20retrieved%20articles.%20Every%20included%20study%20was%20to%20report%20risk%20estimates%20with%2095%25%20CIs%20for%20the%20association%20between%20metabolic%20syndrome%20and%20cancer.%5CnRESULTSdWe%20analyzed%20116%20datasets%20from%2043%20articles%2C%20including%2038%2C940%20cases%20of%20cancer.%20In%20cohort%20studies%20in%20men%2C%20the%20presence%20of%20metabolic%20syndrome%20was%20associated%20with%20liver%20%28relative%20risk%201.43%2C%20P%20%2C%200.0001%29%2C%20colorectal%20%281.25%2C%20P%20%2C%200.001%29%2C%20and%20bladder%20cancer%20%281.10%2C%20P%20%3D%200.013%29.%20In%20cohort%20studies%20in%20women%2C%20the%20presence%20of%20metabolic%20syndrome%20was%20associated%20with%20endometrial%20%281.61%2C%20P%20%3D%200.001%29%2C%20pancreatic%20%281.58%2C%20P%20%2C%200.0001%29%2C%20breast%20postmenopausal%20%281.56%2C%20P%20%3D%200.017%29%2C%20rectal%20%281.52%2C%20P%20%3D%200.005%29%2C%20and%20colorectal%20%281.34%2C%20P%20%3D%200.006%29%20cancers.%20Associations%20with%20metabolic%20syndrome%20were%20stronger%20in%20women%20than%20in%20men%20for%20pancreatic%20%28P%20%3D%200.01%29%20and%20rectal%20%28P%20%3D%200.01%29%20cancers.%20Associations%20were%20different%20between%20ethnic%20groups%3A%20we%20recorded%20stronger%20associations%20in%20Asia%20populations%20for%20liver%20cancer%20%28P%20%3D%200.002%29%2C%20in%20European%20populations%20for%20colorectal%20cancer%20in%20women%20%28P%20%3D%200.004%29%2C%20and%20in%20U.S.%20populations%20%28whites%29%20for%20prostate%20cancer%20%28P%20%3D%200.001%29.%5CnCONCLUSIONSdMetabolic%20syndrome%20is%20associated%20with%20increased%20risk%20of%20common%20cancers%3B%20for%20some%20cancers%2C%20the%20risk%20differs%20betweens%20sexes%2C%20populations%2C%20and%20de%5Cufb01nitions%20of%20metabolic%20syndrome.%22%2C%22date%22%3A%222012-11-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.2337%5C%2Fdc12-0336%22%2C%22ISSN%22%3A%220149-5992%2C%201935-5548%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fcare.diabetesjournals.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.2337%5C%2Fdc12-0336%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A26%3A40Z%22%7D%7D%2C%7B%22key%22%3A%22NJAIXK92%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EObesity%20and%20Risk%20of%20Cancer%3A%20An%20Introductory%20Overview%20%7C%20SpringerLink.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Flink.springer.com%5C%2Fchapter%5C%2F10.1007%5C%2F978-3-319-42542-9_1%27%3Ehttps%3A%5C%2F%5C%2Flink.springer.com%5C%2Fchapter%5C%2F10.1007%5C%2F978-3-319-42542-9_1%3C%5C%2Fa%3E.%20Accessed%2030%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Obesity%20and%20Risk%20of%20Cancer%3A%20An%20Introductory%20Overview%20%7C%20SpringerLink%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flink.springer.com%5C%2Fchapter%5C%2F10.1007%5C%2F978-3-319-42542-9_1%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A20%3A58Z%22%7D%7D%2C%7B%22key%22%3A%22V5LNMCKW%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Seeman%20et%20al.%22%2C%22parsedDate%22%3A%222004%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ESeeman%20TE%2C%20Crimmins%20E%2C%20Huang%20M-H%2C%20et%20al%20%282004%29%20Cumulative%20biological%20risk%20and%20socio-economic%20differences%20in%20mortality%3A%20MacArthur%20Studies%20of%20Successful%20Aging.%20Social%20Science%20%26amp%3B%20Medicine%2058%3A1985%26%23x2013%3B1997.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2FS0277-9536%2803%2900402-7%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2FS0277-9536%2803%2900402-7%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Cumulative%20biological%20risk%20and%20socio-economic%20differences%20in%20mortality%3A%20MacArthur%20Studies%20of%20Successful%20Aging%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Teresa%20E%22%2C%22lastName%22%3A%22Seeman%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eileen%22%2C%22lastName%22%3A%22Crimmins%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mei-Hua%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Burton%22%2C%22lastName%22%3A%22Singer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexander%22%2C%22lastName%22%3A%22Bucur%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tara%22%2C%22lastName%22%3A%22Gruenewald%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lisa%20F%22%2C%22lastName%22%3A%22Berkman%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%20B%22%2C%22lastName%22%3A%22Reuben%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%225%5C%2F2004%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2FS0277-9536%2803%2900402-7%22%2C%22ISSN%22%3A%2202779536%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS0277953603004027%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A18%3A50Z%22%7D%7D%2C%7B%22key%22%3A%22HCF2WDQ2%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Pinquart%20and%20S%5Cu00f6rensen%22%2C%22parsedDate%22%3A%222000%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EPinquart%20M%2C%20S%26%23xF6%3Brensen%20S%20%282000%29%20Influences%20of%20socioeconomic%20status%2C%20social%20network%2C%20and%20competence%20on%20subjective%20well-being%20in%20later%20life%3A%20A%20meta-analysis.%20Psychology%20and%20Aging%2015%3A187%26%23x2013%3B224.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1037%5C%2F0882-7974.15.2.187%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1037%5C%2F0882-7974.15.2.187%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Influences%20of%20socioeconomic%20status%2C%20social%20network%2C%20and%20competence%20on%20subjective%20well-being%20in%20later%20life%3A%20A%20meta-analysis.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Martin%22%2C%22lastName%22%3A%22Pinquart%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Silvia%22%2C%22lastName%22%3A%22S%5Cu00f6rensen%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222000%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1037%5C%2F0882-7974.15.2.187%22%2C%22ISSN%22%3A%221939-1498%2C%200882-7974%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.apa.org%5C%2Fgetdoi.cfm%3Fdoi%3D10.1037%5C%2F0882-7974.15.2.187%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A18%3A43Z%22%7D%7D%2C%7B%22key%22%3A%22JT22QG53%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Orzack%20et%20al.%22%2C%22parsedDate%22%3A%222015-04-21%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EOrzack%20SH%2C%20Stubblefield%20JW%2C%20Akmaev%20VR%2C%20et%20al%20%282015%29%20The%20human%20sex%20ratio%20from%20conception%20to%20birth.%20Proc%20Natl%20Acad%20Sci%20USA%20112%3AE2102%26%23x2013%3BE2111.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1416546112%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1416546112%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20human%20sex%20ratio%20from%20conception%20to%20birth%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Steven%20Hecht%22%2C%22lastName%22%3A%22Orzack%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20William%22%2C%22lastName%22%3A%22Stubblefield%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Viatcheslav%20R.%22%2C%22lastName%22%3A%22Akmaev%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pere%22%2C%22lastName%22%3A%22Colls%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Santiago%22%2C%22lastName%22%3A%22Munn%5Cu00e9%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Scholl%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%22%2C%22lastName%22%3A%22Steinsaltz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22James%20E.%22%2C%22lastName%22%3A%22Zuckerman%22%7D%5D%2C%22abstractNote%22%3A%22We%20describe%20the%20trajectory%20of%20the%20human%20sex%20ratio%20from%20conception%20to%20birth%20by%20analyzing%20data%20from%20%28%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20i%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20%29%203-%20to%206-d-old%20embryos%2C%20%28%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20ii%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20%29%20induced%20abortions%2C%20%28%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20iii%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20%29%20chorionic%20villus%20sampling%2C%20%28%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20iv%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20%29%20amniocentesis%2C%20and%20%28%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20v%5Cn%20%20%20%20%20%20%20%20%20%20%20%20%20%20%29%20fetal%20deaths%20and%20live%20births.%20Our%20dataset%20is%20the%20most%20comprehensive%20and%20largest%20ever%20assembled%20to%20estimate%20the%20sex%20ratio%20at%20conception%20and%20the%20sex%20ratio%20trajectory%20and%20is%20the%20first%2C%20to%20our%20knowledge%2C%20to%20include%20all%20of%20these%20types%20of%20data.%20Our%20estimate%20of%20the%20sex%20ratio%20at%20conception%20is%200.5%20%28proportion%20male%29%2C%20which%20contradicts%20the%20common%20claim%20that%20the%20sex%20ratio%20at%20conception%20is%20male-biased.%20The%20sex%20ratio%20among%20abnormal%20embryos%20is%20male-biased%2C%20and%20the%20sex%20ratio%20among%20normal%20embryos%20is%20female-biased.%20These%20biases%20are%20associated%20with%20the%20abnormal%5C%2Fnormal%20state%20of%20the%20sex%20chromosomes%20and%20of%20chromosomes%2015%20and%2017.%20The%20sex%20ratio%20may%20decrease%20in%20the%20first%20week%20or%20so%20after%20conception%20%28due%20to%20excess%20male%20mortality%29%3B%20it%20then%20increases%20for%20at%20least%2010%5Cu201315%20wk%20%28due%20to%20excess%20female%20mortality%29%2C%20levels%20off%20after%20%5Cu223c20%20wk%2C%20and%20declines%20slowly%20from%2028%20to%2035%20wk%20%28due%20to%20excess%20male%20mortality%29.%20Total%20female%20mortality%20during%20pregnancy%20exceeds%20total%20male%20mortality.%20The%20unbiased%20sex%20ratio%20at%20conception%2C%20the%20increase%20in%20the%20sex%20ratio%20during%20the%20first%20trimester%2C%20and%20total%20mortality%20during%20pregnancy%20being%20greater%20for%20females%20are%20fundamental%20insights%20into%20early%20human%20development.%22%2C%22date%22%3A%222015-04-21%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1073%5C%2Fpnas.1416546112%22%2C%22ISSN%22%3A%220027-8424%2C%201091-6490%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.pnas.org%5C%2Flookup%5C%2Fdoi%5C%2F10.1073%5C%2Fpnas.1416546112%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A06%3A36Z%22%7D%7D%2C%7B%22key%22%3A%22EG2K9KAT%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Austad%22%2C%22parsedDate%22%3A%222015-04-21%22%2C%22numChildren%22%3A3%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EAustad%20SN%20%282015%29%20The%20human%20prenatal%20sex%20ratio%3A%20A%20major%20surprise.%20PNAS%20112%3A4839%26%23x2013%3B4840.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1505165112%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1505165112%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20human%20prenatal%20sex%20ratio%3A%20A%20major%20surprise%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Steven%20N.%22%2C%22lastName%22%3A%22Austad%22%7D%5D%2C%22abstractNote%22%3A%22Two%20remarkably%20consistent%20and%20poorly%20understood%20features%20of%20human%20biology%20are%20the%20slightly%20male-biased%20sex%20ratio%20at%20birth%20and%20the%20female%20survival%20advantage%20throughout%20life.%20These%20patterns%20appear%20across%20geography%20and%20time%20wherever%20reliable%20birth%20and%20death%20records%20are%20available%20%281%2C%202%29.%20The%20slight%20male%20bias%2C%20typically%20%5Cu223c51.3%25%20of%20live%20births%2C%20is%20so%20consistent%20%28Fig.%201%20A%20%29%20that%20when%20birth%20sex%20ratios%20deviate%20much%20from%20it%2C%20suspicions%20are%20aroused%20of%20sex-specific%20abortion%20or%20infanticide%20%283%2C%204%29.%20Putting%20together%20the%20birth%20sex%20ratio%20bias%20and%20the%20female%20survival%20advantage%20%285%29%2C%20we%20expect%20a%20monotonically%20declining%20sex%20ratio%20from%20birth%20to%20death%2C%20which%20is%20exactly%20what%20we%20find%20across%20cultures%20and%20across%20historical%20epochs%20%28Fig.%201%20B%20%29.%20By%20age%20100%20y%2C%20there%20are%20three%20to%20four%20women%20for%20every%20surviving%20man%2C%20and%20by%20the%20extraordinary%20age%20of%20110%20y%2C%2095%25%20of%20the%20survivors%20are%20women.%20Note%2C%20however%2C%20that%20until%20later%20life%20the%20sex%20ratio%20does%20not%20stray%20far%20from%2050%3A50%2C%20an%20observation%20that%20would%20gladden%20the%20heart%20of%20evolutionary%20biologist%2C%20Sir%20Ronald%20Fisher%2C%20who%20argued%20that%20natural%20selection%20should%20favor%20equal%20parental%20expenditure%5Cu2014a%20delightfully%20vague%20phrase%5Cu2014in%20males%20and%20females%20%286%29.%20Fisher%20assumed%2C%20as%20have%20many%20since%20then%2C%20that%20the%20human%20sex%20ratio%20at%20conception%20is%20even%20more%20male-biased%20than%20the%20sex%20ratio%20at%20birth%2C%20and%20there%20were%20some%20good%20reasons%20to%20assume%20this.%20First%2C%20males%20are%20less%20likely%20%5Cu2026%20%5Cn%5Cn%5B%5Cu21b5%5D%5B1%5D1Email%3A%20austad%7Bat%7Duab.edu.%5Cn%5Cn%20%5B1%5D%3A%20%23xref-corresp-1-1%22%2C%22date%22%3A%222015%5C%2F04%5C%2F21%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1073%5C%2Fpnas.1505165112%22%2C%22ISSN%22%3A%220027-8424%2C%201091-6490%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.pnas.org%5C%2Fcontent%5C%2F112%5C%2F16%5C%2F4839%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A05%3A43Z%22%7D%7D%2C%7B%22key%22%3A%2298E6DTMD%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Austad%20and%20Fischer%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EAustad%20SN%2C%20Fischer%20KE%20%282016%29%20Sex%20Differences%20in%20Lifespan.%20Cell%20Metabolism%2023%3A1022%26%23x2013%3B1033.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cmet.2016.05.019%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cmet.2016.05.019%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Sex%20Differences%20in%20Lifespan%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Steven%20N.%22%2C%22lastName%22%3A%22Austad%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kathleen%20E.%22%2C%22lastName%22%3A%22Fischer%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2206%5C%2F2016%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.cmet.2016.05.019%22%2C%22ISSN%22%3A%2215504131%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1550413116302376%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T10%3A00%3A37Z%22%7D%7D%2C%7B%22key%22%3A%224S3DHHQN%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lema%5Cu00eetre%20et%20al.%22%2C%22parsedDate%22%3A%222020-03-23%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ELema%26%23xEE%3Btre%20J-F%2C%20Ronget%20V%2C%20Tidi%26%23xE8%3Bre%20M%2C%20et%20al%20%282020%29%20Sex%20differences%20in%20adult%20lifespan%20and%20aging%20rates%20of%20mortality%20across%20wild%20mammals.%20Proc%20Natl%20Acad%20Sci%20USA.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1911999117%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1911999117%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Sex%20differences%20in%20adult%20lifespan%20and%20aging%20rates%20of%20mortality%20across%20wild%20mammals%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Fran%5Cu00e7ois%22%2C%22lastName%22%3A%22Lema%5Cu00eetre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Victor%22%2C%22lastName%22%3A%22Ronget%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Morgane%22%2C%22lastName%22%3A%22Tidi%5Cu00e8re%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dominique%22%2C%22lastName%22%3A%22Allain%5Cu00e9%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22V%5Cu00e9rane%22%2C%22lastName%22%3A%22Berger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aur%5Cu00e9lie%22%2C%22lastName%22%3A%22Cohas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fernando%22%2C%22lastName%22%3A%22Colchero%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dalia%20A.%22%2C%22lastName%22%3A%22Conde%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%22%2C%22lastName%22%3A%22Garratt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andr%5Cu00e1s%22%2C%22lastName%22%3A%22Liker%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gabriel%20A.%20B.%22%2C%22lastName%22%3A%22Marais%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexander%22%2C%22lastName%22%3A%22Scheuerlein%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tam%5Cu00e1s%22%2C%22lastName%22%3A%22Sz%5Cu00e9kely%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Michel%22%2C%22lastName%22%3A%22Gaillard%22%7D%5D%2C%22abstractNote%22%3A%22In%20human%20populations%2C%20women%20consistently%20outlive%20men%2C%20which%20suggests%20profound%20biological%20foundations%20for%20sex%20differences%20in%20survival.%20Quantifying%20whether%20such%20sex%20differences%20are%20also%20pervasive%20in%20wild%20mammals%20is%20a%20crucial%20challenge%20in%20both%20evolutionary%20biology%20and%20biogerontology.%20Here%2C%20we%20compile%20demographic%20data%20from%20134%20mammal%20populations%2C%20encompassing%20101%20species%2C%20to%20show%20that%20the%20female%27s%20median%20lifespan%20is%20on%20average%2018.6%25%20longer%20than%20that%20of%20conspecific%20males%2C%20whereas%20in%20humans%20the%20female%20advantage%20is%20on%20average%207.8%25.%20On%20the%20contrary%2C%20we%20do%20not%20find%20any%20consistent%20sex%20differences%20in%20aging%20rates.%20In%20addition%2C%20sex%20differences%20in%20median%20adult%20lifespan%20and%20aging%20rates%20are%20both%20highly%20variable%20across%20species.%20Our%20analyses%20suggest%20that%20the%20magnitude%20of%20sex%20differences%20in%20mammalian%20mortality%20patterns%20is%20likely%20shaped%20by%20local%20environmental%20conditions%20in%20interaction%20with%20the%20sex-specific%20costs%20of%20sexual%20selection.%22%2C%22date%22%3A%22Mar%2023%2C%202020%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1073%5C%2Fpnas.1911999117%22%2C%22ISSN%22%3A%221091-6490%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-30T09%3A47%3A09Z%22%7D%7D%2C%7B%22key%22%3A%22B6QAFUMT%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zanoboni%20et%20al.%22%2C%22parsedDate%22%3A%221976-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EZanoboni%20A%2C%20Schwarz%20D%2C%20Zanoboni-Muciaccia%20W%20%281976%29%20Stimulation%20of%20insulin%20secretion%20in%20man%20by%20oral%20glycerol%20administration.%20Metab%20Clin%20Exp%2025%3A41%26%23x2013%3B45.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2F0026-0495%2876%2990158-x%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2F0026-0495%2876%2990158-x%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Stimulation%20of%20insulin%20secretion%20in%20man%20by%20oral%20glycerol%20administration%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Zanoboni%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22D.%22%2C%22lastName%22%3A%22Schwarz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22W.%22%2C%22lastName%22%3A%22Zanoboni-Muciaccia%22%7D%5D%2C%22abstractNote%22%3A%22The%20effects%20of%20an%20orally%20administered%20glycerol%20load%20%281%20g%5C%2FKg%20body%20weight%29%20on%20blood%20glucose%2C%20plasma%20FFA%2C%20and%20plasma%20insulin%20levels%20have%20been%20determined%20in%20eight%20normal%20fasting%20or%20glucose%20loaded%20%281%20g%5C%2FKg%20body%20weight%29%20volunteers.%20Blood%20glucose%20levels%20were%20not%20affected%20by%20glycerol%20loading%20while%20glicemia%20followed%20the%20same%20pattern%20of%20a%20glucose%20tolerance%20test%20in%20the%20group%20treated%20with%20glucose%20plus%20glycerol.%20Plasma%20FFA%20were%20significantly%20lowered%20only%2090%20min%20after%20glycerol%20loading%20while%20they%20had%20markedly%20and%20persistently%20decreased%20by%20glycerol%20plus%20glucose%20per%20os.%20Finally%2C%20though%20glicemia%20did%20not%20change%2C%20insulinemia%20was%20markedly%20increased%20by%20glycereol%2C%2090%20min%20after%20loading%3B%20moreover%2C%20plasma%20IRI%20was%20significantly%20higher%20in%20the%20group%20treated%20with%20glycerol%20plus%20glucose%20than%20in%20the%20group%20treated%20with%20glucose%20alone.%20These%20data%20suggest%20that%20the%20release%20of%20insulin%20may%20be%20stimulated%20by%20a%20very%20small%20increment%20of%20blood%20glucose%2C%20which%20derives%20from%20glycerol.%22%2C%22date%22%3A%22Jan%201976%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1016%5C%2F0026-0495%2876%2990158-x%22%2C%22ISSN%22%3A%220026-0495%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%226ZD5MYAL%22%5D%2C%22dateModified%22%3A%222020-03-29T14%3A53%3A22Z%22%7D%7D%2C%7B%22key%22%3A%22KC29XC9H%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EAssociation%20of%20Triglyceride-Lowering%20LPL%20Variants%20and%20LDL-C%26%23x2013%3BLowering%20LDLR%20Variants%20With%20Risk%20of%20Coronary%20Heart%20Disease%20%7C%20Cardiology%20%7C%20JAMA%20%7C%20JAMA%20Network.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fjamanetwork.com%5C%2Fjournals%5C%2Fjama%5C%2Farticle-abstract%5C%2F2722770%27%3Ehttps%3A%5C%2F%5C%2Fjamanetwork.com%5C%2Fjournals%5C%2Fjama%5C%2Farticle-abstract%5C%2F2722770%3C%5C%2Fa%3E.%20Accessed%2029%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Association%20of%20Triglyceride-Lowering%20LPL%20Variants%20and%20LDL-C%5Cu2013Lowering%20LDLR%20Variants%20With%20Risk%20of%20Coronary%20Heart%20Disease%20%7C%20Cardiology%20%7C%20JAMA%20%7C%20JAMA%20Network%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fjamanetwork.com%5C%2Fjournals%5C%2Fjama%5C%2Farticle-abstract%5C%2F2722770%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-29T10%3A39%3A29Z%22%7D%7D%2C%7B%22key%22%3A%22N98JYWCU%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Madsen%20et%20al.%22%2C%22parsedDate%22%3A%222008%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EMadsen%20EL%2C%20Rissanen%20A%2C%20Bruun%20JM%2C%20et%20al%20%282008%29%20Weight%20loss%20larger%20than%2010%25%20is%20needed%20for%20general%20improvement%20of%20levels%20of%20circulating%20adiponectin%20and%20markers%20of%20inflammation%20in%20obese%20subjects%3A%20a%203-year%20weight%20loss%20study.%20European%20Journal%20of%20Endocrinology%20158%3A179%26%23x2013%3B187.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1530%5C%2FEJE-07-0721%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1530%5C%2FEJE-07-0721%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Weight%20loss%20larger%20than%2010%25%20is%20needed%20for%20general%20improvement%20of%20levels%20of%20circulating%20adiponectin%20and%20markers%20of%20inflammation%20in%20obese%20subjects%3A%20a%203-year%20weight%20loss%20study%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Erik%20L%22%2C%22lastName%22%3A%22Madsen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aila%22%2C%22lastName%22%3A%22Rissanen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jens%20M%22%2C%22lastName%22%3A%22Bruun%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kristin%22%2C%22lastName%22%3A%22Skogstrand%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Serena%22%2C%22lastName%22%3A%22Tonstad%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%20M%22%2C%22lastName%22%3A%22Hougaard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bj%5Cu00f8rn%22%2C%22lastName%22%3A%22Richelsen%22%7D%5D%2C%22abstractNote%22%3A%22Objective%3A%20To%20investigate%20the%20effects%20of%3A%20I%29%20short-%20%288%20weeks%29%2C%20II%29%20long-term%20%283%20years%29%20weight%20loss%2C%20and%20III%29%20the%20degree%20of%20weight%20loss%20on%20circulating%20levels%20of%20adiponectin%2C%20high%20sensitive-C%20reactive%20protein%20%28hs-CRP%29%2C%20and%20%5Cufb01brinogen%20in%20obese%20subjects.%20Moreover%2C%20to%20evaluate%20the%20effect%20of%20the%20lipase%20inhibitor%2C%20orlistat%2C%20on%20these%20parameters.%20Design%3A%20Weight%20loss%20induced%20in%2093%20obese%20subjects%20%28mean%20weight%3A%20108.9G15.8%20kg%29%20through%208-week%20very-low-energy%20diet%20%28VLED%2C%20800%20kcal%5C%2Fday%29%20followed%20by%20randomization%20to%20orlistat%20or%20placebo%20together%20with%20lifestyle%20intervention%20for%20further%203%20years.%20Adiponectin%20and%20hs-CRP%20were%20measured%20at%20baseline%2C%20after%208%20weeks%20of%20VLED%20and%206%2C%2012%2C%20and%2036%20months%20after%20the%20VLED%20by%20%5Cufb02owmetric%20xMAP%20technology%20%28Luminex%20Multi-Analyte%20Pro%5Cufb01ling%20System%2C%20Luminex%20Corp.%2C%20Austin%2C%20TX%2C%20USA%29.%20Fibrinogen%20was%20measured%20in%20a%20coagulation%20assay.%5CnResults%3A%20Weight%20loss%20after%20VLED%20treatment%20was%2014.3G4.5%20kg%20and%20after%203%20years%207.7G8.7%20kg.%20Orlistattreated%20subjects%20regained%203.9%20kg%20less%20than%20placebo-treated%20from%20the%20end%20of%20the%20VLED%20to%203%20years%20%28PZ0.01%29.%20No%20differences%20were%20detected%20between%20the%20two%20groups%20regarding%20changes%20in%20adiponectin%2C%20hs-CRP%2C%20or%20%5Cufb01brinogen.%20Accordingly%2C%20the%20groups%20were%20combined%20for%20further%20analyses.%20Serum%20adiponectin%20increased%20by%2022%25%20%28P%210.05%29%20after%20the%20VLED%20but%20returned%20to%20baseline%20after%203%20years.%20Both%20short-%20and%20long-term%20weight%20losses%20needed%20to%20be%20in%20excess%20of%2010%25%20%28w12%20kg%29%20in%20order%20to%20increase%20adiponectin%20levels%20signi%5Cufb01cantly.%20Weight%20loss%20was%20associated%20with%20a%20signi%5Cufb01cant%20decrease%20in%20hs-CRP.%20Fibrinogen%20decreased%20by%2012%25%20%28P%210.05%29%20after%203%20years.%5CnConclusions%3A%20In%20obese%20subjects%2C%20weight%20loss%20was%20associated%20with%20an%20increase%20in%20serum%20adiponectin%20and%20a%20decrease%20in%20hs-CRP%20and%20plasma%20%5Cufb01brinogen.%20Long-term%20weight%20loss%20%283%20years%29%20must%20exceed%2010%25%20to%20induce%20a%20combined%20signi%5Cufb01cant%20improvement%20in%20these%20in%5Cufb02ammatory%20markers.%22%2C%22date%22%3A%2202%5C%2F2008%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1530%5C%2FEJE-07-0721%22%2C%22ISSN%22%3A%220804-4643%2C%201479-683X%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Feje.bioscientifica.com%5C%2Fview%5C%2Fjournals%5C%2Feje%5C%2F158%5C%2F2%5C%2F179.xml%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-26T18%3A25%3A24Z%22%7D%7D%2C%7B%22key%22%3A%22ZAHCKML2%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Danesh%20et%20al.%22%2C%22parsedDate%22%3A%222000-07-22%22%2C%22numChildren%22%3A3%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EDanesh%20J%2C%20Whincup%20P%2C%20Walker%20M%2C%20et%20al%20%282000%29%20Low%20grade%20inflammation%20and%20coronary%20heart%20disease%3A%20prospective%20study%20and%20updated%20meta-analyses.%20BMJ%20321%3A199%26%23x2013%3B204.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1136%5C%2Fbmj.321.7255.199%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1136%5C%2Fbmj.321.7255.199%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Low%20grade%20inflammation%20and%20coronary%20heart%20disease%3A%20prospective%20study%20and%20updated%20meta-analyses%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%22%2C%22lastName%22%3A%22Danesh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Peter%22%2C%22lastName%22%3A%22Whincup%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mary%22%2C%22lastName%22%3A%22Walker%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lucy%22%2C%22lastName%22%3A%22Lennon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrew%22%2C%22lastName%22%3A%22Thomson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paul%22%2C%22lastName%22%3A%22Appleby%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20Ruth%22%2C%22lastName%22%3A%22Gallimore%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mark%20B.%22%2C%22lastName%22%3A%22Pepys%22%7D%5D%2C%22abstractNote%22%3A%22Objective%3A%20To%20assess%20associations%20between%20baseline%20values%20of%20four%20different%20circulating%20markers%20of%20inflammation%20and%20future%20risk%20of%20coronary%20heart%20disease%2C%20potential%20triggers%20of%20systemic%20inflammation%20%28such%20as%20persistent%20infection%29%2C%20and%20other%20markers%20of%20inflammation.%5CnDesign%3A%20Nested%20case-control%20comparisons%20in%20a%20prospective%2C%20population%20based%20cohort.%5CnSetting%3A%20General%20practices%20in%2018%20towns%20in%20Britain.%5CnParticipants%3A%20506%20men%20who%20died%20from%20coronary%20heart%20disease%20or%20had%20a%20non-fatal%20myocardial%20infarction%20and%201025%20men%20who%20remained%20free%20of%20such%20disease%20until%201996%20selected%20from%205661%20men%20aged%2040%5Cu201359%20years%20who%20provided%20blood%20samples%20in%201978-1980.%5CnMain%20outcome%20measures%3A%20Plasma%20concentrations%20of%20C%20reactive%20protein%2C%20serum%20amyloid%20A%20protein%2C%20and%20serum%20albumin%20and%20leucocyte%20count.%20Information%20on%20fatal%20and%20non-fatal%20coronary%20heart%20disease%20was%20obtained%20from%20medical%20records%20and%20death%20certificates.%5CnResults%3A%20Compared%20with%20men%20in%20the%20bottom%20third%20of%20baseline%20measurements%20of%20C%20reactive%20protein%2C%20men%20in%20the%20top%20third%20had%20an%20odds%20ratio%20for%20coronary%20heart%20disease%20of%202.13%20%2895%25%20confidence%20interval%201.38%20to%203.28%29%20after%20age%2C%20town%2C%20smoking%2C%20vascular%20risk%20factors%2C%20and%20indicators%20of%20socioeconomic%20status%20were%20adjusted%20for.%20Similar%20adjusted%20odds%20ratios%20were%201.65%20%281.07%20to%202.55%29%20for%20serum%20amyloid%20A%20protein%3B%201.12%20%280.71%20to%201.77%29%20for%20leucocyte%20count%3B%20and%200.67%20%280.43%20to%201.04%29%20for%20albumin.%20No%20strong%20associations%20were%20observed%20of%20these%20factors%20with%20Helicobacter%20pylori%20seropositivity%2C%20Chlamydia%20pneumoniae%20IgG%20titres%2C%20or%20plasma%20total%20homocysteine%20concentrations.%20Baseline%20values%20of%20the%20acute%20phase%20reactants%20were%20significantly%20associated%20with%20one%20another%20%28P%3C0.0001%29%2C%20although%20the%20association%20between%20low%20serum%20albumin%20concentration%20and%20leucocyte%20count%20was%20weaker%20%28P%3D0.08%29.%5CnConclusion%3A%20In%20the%20context%20of%20results%20from%20other%20relevant%20studies%20these%20findings%20suggest%20that%20some%20inflammatory%20processes%2C%20unrelated%20to%20the%20chronic%20infections%20studied%20here%2C%20are%20likely%20to%20be%20involved%20in%20coronary%20heart%20disease.%22%2C%22date%22%3A%222000%5C%2F07%5C%2F22%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1136%5C%2Fbmj.321.7255.199%22%2C%22ISSN%22%3A%220959-8138%2C%201468-5833%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.bmj.com%5C%2Fcontent%5C%2F321%5C%2F7255%5C%2F199%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-26T18%3A13%3A11Z%22%7D%7D%2C%7B%22key%22%3A%22BZZFC7M4%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Nguyen%20et%20al.%22%2C%22parsedDate%22%3A%222010-09-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ENguyen%20NT%2C%20Nguyen%20X-MT%2C%20Wooldridge%20JB%2C%20et%20al%20%282010%29%20Association%20of%20obesity%20with%20risk%20of%20coronary%20heart%20disease%3A%20findings%20from%20the%20National%20Health%20and%20Nutrition%20Examination%20Survey%2C%201999%26%23x2013%3B2006.%20Surgery%20for%20Obesity%20and%20Related%20Diseases%206%3A465%26%23x2013%3B469.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.soard.2010.02.038%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.soard.2010.02.038%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Association%20of%20obesity%20with%20risk%20of%20coronary%20heart%20disease%3A%20findings%20from%20the%20National%20Health%20and%20Nutrition%20Examination%20Survey%2C%201999%5Cu20132006%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ninh%20T.%22%2C%22lastName%22%3A%22Nguyen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xuan-Mai%20T.%22%2C%22lastName%22%3A%22Nguyen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22James%20B.%22%2C%22lastName%22%3A%22Wooldridge%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Johnathan%20A.%22%2C%22lastName%22%3A%22Slone%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%20S.%22%2C%22lastName%22%3A%22Lane%22%7D%5D%2C%22abstractNote%22%3A%22Background%5CnObesity%20is%20a%20well-known%20risk%20factor%20for%20the%20development%20of%20coronary%20heart%20disease%20%28CHD%29.%20The%20aim%20of%20the%20present%20study%20was%20to%20examine%20the%20differences%20in%20the%2010-year%20CHD%20risk%20with%20increasing%20severity%20of%20obesity%20in%20men%20and%20women%20participating%20in%20the%20latest%20National%20Health%20and%20Nutrition%20Examination%20Survey.%5CnMethods%5CnData%20from%20a%20representative%20sample%20of%2012%2C500%20U.S.%20participants%20in%20the%20National%20Health%20and%20Nutrition%20Examination%20Survey%20from%201999%20to%202006%20were%20reviewed.%20The%20Framingham%20risk%20score%20was%20calculated%20for%20men%20and%20women%20according%20to%20a%20body%20mass%20index%20%28BMI%29%20of%20%3C25.0%2C%2025.0%5Cu201329.9%2C%2030.0%5Cu201334.9%2C%20and%20%5Cu226535.0%20kg%5C%2Fm2.%5CnResults%5CnThe%20prevalence%20of%20those%20with%20hypertension%20increased%20with%20an%20increasing%20BMI%2C%20from%2024%25%20for%20a%20BMI%20%3C25.0%20kg%5C%2Fm2%20to%2054%25%20for%20a%20BMI%20of%20%5Cu226535.0%20kg%5C%2Fm2.%20The%20prevalence%20of%20an%20abnormal%20total%20cholesterol%20level%20%28%3E200%20mg%5C%2FdL%29%20increased%20from%2040%25%20for%20a%20BMI%20%3C25.0%20kg%5C%2Fm2%20to%2048%25%20for%20a%20BMI%20of%20%5Cu226535.0%20kg%5C%2Fm2.%20The%2010-year%20CHD%20risk%20for%20men%20increased%20from%203.1%25%20for%20a%20BMI%20%3C25.0%20kg%5C%2Fm2%20to%20a%20peak%20of%205.6%25%20for%20a%20BMI%20of%2030.0%5Cu201334.9%20kg%5C%2Fm2.%20The%2010-year%20CHD%20risk%20for%20women%20increased%20from%20.8%25%20for%20a%20BMI%20%3C25.0%20kg%5C%2Fm2%20to%20a%20peak%20of%201.5%25%20for%20a%20BMI%20of%20%5Cu226535.0%20kg%5C%2Fm2.%20Both%20diabetes%20and%20hypertension%20were%20independent%20risk%20factors%20for%20an%20increasing%20CHD%20risk.%5CnConclusions%5CnThe%2010-year%20CHD%20risk%2C%20calculated%20using%20the%20Framingham%20risk%20score%2C%20substantially%20increased%20with%20an%20increasing%20BMI.%20An%20important%20implication%20from%20our%20findings%20is%20the%20need%20to%20implement%20surgical%20and%20medical%20approaches%20to%20weight%20reduction%20to%20reduce%20the%20effect%20of%20morbidity%20and%20mortality%20from%20CHD%20on%20the%20U.S.%20healthcare%20system.%22%2C%22date%22%3A%22September%201%2C%202010%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.soard.2010.02.038%22%2C%22ISSN%22%3A%221550-7289%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS155072891000078X%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-26T18%3A06%3A04Z%22%7D%7D%2C%7B%22key%22%3A%226QAPJYLP%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Partsalaki%20et%20al.%22%2C%22parsedDate%22%3A%222012%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EPartsalaki%20I%2C%20Karvela%20A%2C%20Spiliotis%20BE%20%282012%29%20Metabolic%20impact%20of%20a%20ketogenic%20diet%20compared%20to%20a%20hypocaloric%20diet%20in%20obese%20children%20and%20adolescents.%20J%20Pediatr%20Endocrinol%20Metab%2025%3A697%26%23x2013%3B704.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1515%5C%2Fjpem-2012-0131%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1515%5C%2Fjpem-2012-0131%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Metabolic%20impact%20of%20a%20ketogenic%20diet%20compared%20to%20a%20hypocaloric%20diet%20in%20obese%20children%20and%20adolescents%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ioanna%22%2C%22lastName%22%3A%22Partsalaki%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexia%22%2C%22lastName%22%3A%22Karvela%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bessie%20E.%22%2C%22lastName%22%3A%22Spiliotis%22%7D%5D%2C%22abstractNote%22%3A%22BACKGROUND%3A%20The%20effects%20of%20carbohydrate-restricted%20%28ketogenic%29%20diets%20on%20metabolic%20parameters%20in%20children%20have%20been%20incompletely%20assessed.%5CnOBJECTIVE%3A%20To%20compare%20the%20efficacy%20and%20metabolic%20impact%20of%20ketogenic%20and%20hypocaloric%20diets%20in%20obese%20children%20and%20adolescents.%5CnSUBJECTS%3A%20Fifty-eight%20obese%20subjects%20were%20placed%20on%20one%20of%20the%20two%20diets%20for%206%20months.%5CnMETHODS%3A%20Anthropometric%20measurements%2C%20body%20composition%2C%20oral%20glucose%5C%2Finsulin%20tolerance%20test%2C%20lipidemic%20profile%2C%20high%20molecular%20weight%20%28HMW%29%20adiponectin%2C%20whole-body%20insulin%20sensitivity%20index%20%28WBISI%29%2C%20and%20homeostatic%20model%20assessment-insulin%20resistance%20%28HOMA-IR%29%20were%20determined%20before%20and%20after%20each%20diet.%5CnRESULTS%3A%20Both%20groups%20significantly%20reduced%20their%20weight%2C%20fat%20mass%2C%20waist%20circumference%2C%20fasting%20insulin%2C%20and%20HOMA-IR%20%28p%20%3D%200.009%20for%20ketogenic%20and%20p%20%3D%200.014%20for%20hypocaloric%29%2C%20but%20the%20differences%20were%20greater%20in%20the%20ketogenic%20group.%20Both%20groups%20increased%20WBISI%20significantly%2C%20but%20only%20the%20ketogenic%20group%20increased%20HMW%20adiponectin%20significantly%20%28p%20%3D%200.025%29.%5CnCONCLUSIONS%3A%20The%20ketogenic%20diet%20revealed%20more%20pronounced%20improvements%20in%20weight%20loss%20and%20metabolic%20parameters%20than%20the%20hypocaloric%20diet%20and%20may%20be%20a%20feasible%20and%20safe%20alternative%20for%20children%27s%20weight%20loss.%22%2C%22date%22%3A%222012%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1515%5C%2Fjpem-2012-0131%22%2C%22ISSN%22%3A%220334-018X%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222020-03-26T18%3A01%3A30Z%22%7D%7D%2C%7B%22key%22%3A%2269H39AXY%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Volek%20et%20al.%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EVolek%20J%2C%20Phinney%20S%2C%20Forsythe%20C%2C%20et%20al%20%283%29%20%28PDF%29%20Carbohydrate%20Restriction%20has%20a%20More%20Favorable%20Impact%20on%20the%20Metabolic%20Syndrome%20than%20a%20Low%20Fat%20Diet.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.researchgate.net%5C%2Fpublication%5C%2F23663658_Carbohydrate_Restriction_has_a_More_Favorable_Impact_on_the_Metabolic_Syndrome_than_a_Low_Fat_Diet%27%3Ehttps%3A%5C%2F%5C%2Fwww.researchgate.net%5C%2Fpublication%5C%2F23663658_Carbohydrate_Restriction_has_a_More_Favorable_Impact_on_the_Metabolic_Syndrome_than_a_Low_Fat_Diet%3C%5C%2Fa%3E.%20Accessed%2026%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22%283%29%20%28PDF%29%20Carbohydrate%20Restriction%20has%20a%20More%20Favorable%20Impact%20on%20the%20Metabolic%20Syndrome%20than%20a%20Low%20Fat%20Diet%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J%22%2C%22lastName%22%3A%22Volek%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephen%22%2C%22lastName%22%3A%22Phinney%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cassandra%22%2C%22lastName%22%3A%22Forsythe%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Erin%22%2C%22lastName%22%3A%22Quinn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%22%2C%22lastName%22%3A%22Wood%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%22%2C%22lastName%22%3A%22Puglisi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22William%20J%22%2C%22lastName%22%3A%22Kraemer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Doug%22%2C%22lastName%22%3A%22Bibus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Maria%20Luz%22%2C%22lastName%22%3A%22Fernandez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%20D%22%2C%22lastName%22%3A%22Feinman%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.researchgate.net%5C%2Fpublication%5C%2F23663658_Carbohydrate_Restriction_has_a_More_Favorable_Impact_on_the_Metabolic_Syndrome_than_a_Low_Fat_Diet%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22TX7VNSFI%22%5D%2C%22dateModified%22%3A%222020-03-26T17%3A58%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22S7I9MHTC%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EKeto%20Longevity%20Webinar.%20In%3A%20Google%20Docs.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdocs.google.com%5C%2Fdocument%5C%2Fu%5C%2F1%5C%2Fd%5C%2F1uD7YzT94lPqTYB00DGyKEDuQNjp0TOKKGv5d1s6dbdI%5C%2Fedit%3Fusp%3Dsharing%26usp%3Dembed_facebook%27%3Ehttps%3A%5C%2F%5C%2Fdocs.google.com%5C%2Fdocument%5C%2Fu%5C%2F1%5C%2Fd%5C%2F1uD7YzT94lPqTYB00DGyKEDuQNjp0TOKKGv5d1s6dbdI%5C%2Fedit%3Fusp%3Dsharing%26usp%3Dembed_facebook%3C%5C%2Fa%3E.%20Accessed%2026%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Keto%20Longevity%20Webinar%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Title%3A%20Can%20You%20Extend%20Your%20Lifespan%20With%20a%20Ketogenic%20Diet%3F%20%20Intro%3A%20The%20Guaranteed%20Ketogenic%20Diet%20%20Part%201%3A%20How%20Long%20Can%20You%20Expect%20to%20Live%3F%20How%20long%20does%20the%20average%20person%20live%3F%20-%20Erin%20Gender%20differences%20Location%20How%20much%20influenced%20by%20diet%20itself%3F%20Socioeconomic%3F%20How%20much%20can%20we%20impact%3F%20Genetics%3F...%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdocs.google.com%5C%2Fdocument%5C%2Fu%5C%2F1%5C%2Fd%5C%2F1uD7YzT94lPqTYB00DGyKEDuQNjp0TOKKGv5d1s6dbdI%5C%2Fedit%3Fusp%3Dsharing%26usp%3Dembed_facebook%22%2C%22language%22%3A%22en-GB%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222020-03-26T17%3A28%3A14Z%22%7D%7D%2C%7B%22key%22%3A%22W7HE8HHJ%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Wilson%20et%20al.%22%2C%22parsedDate%22%3A%222005-11-15%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EWilson%20PWF%2C%20D%26%23x2019%3BAgostino%20RB%2C%20Parise%20H%2C%20et%20al%20%282005%29%20Metabolic%20Syndrome%20as%20a%20Precursor%20of%20Cardiovascular%20Disease%20and%20Type%202%20Diabetes%20Mellitus.%20Circulation%20112%3A3066%26%23x2013%3B3072.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1161%5C%2FCIRCULATIONAHA.105.539528%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1161%5C%2FCIRCULATIONAHA.105.539528%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Metabolic%20Syndrome%20as%20a%20Precursor%20of%20Cardiovascular%20Disease%20and%20Type%202%20Diabetes%20Mellitus%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Peter%20W.F.%22%2C%22lastName%22%3A%22Wilson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ralph%20B.%22%2C%22lastName%22%3A%22D%5Cu2019Agostino%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Helen%22%2C%22lastName%22%3A%22Parise%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lisa%22%2C%22lastName%22%3A%22Sullivan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22James%20B.%22%2C%22lastName%22%3A%22Meigs%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222005-11-15%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1161%5C%2FCIRCULATIONAHA.105.539528%22%2C%22ISSN%22%3A%220009-7322%2C%201524-4539%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ahajournals.org%5C%2Fdoi%5C%2F10.1161%5C%2FCIRCULATIONAHA.105.539528%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222020-03-26T17%3A09%3A36Z%22%7D%7D%2C%7B%22key%22%3A%22T65RIIYX%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Gershuni%20et%20al.%22%2C%22parsedDate%22%3A%222018-09-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EGershuni%20VM%2C%20Yan%20SL%2C%20Medici%20V%20%282018%29%20Nutritional%20Ketosis%20for%20Weight%20Management%20and%20Reversal%20of%20Metabolic%20Syndrome.%20Curr%20Nutr%20Rep%207%3A97%26%23x2013%3B106.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs13668-018-0235-0%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs13668-018-0235-0%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Nutritional%20Ketosis%20for%20Weight%20Management%20and%20Reversal%20of%20Metabolic%20Syndrome%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Victoria%20M.%22%2C%22lastName%22%3A%22Gershuni%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephanie%20L.%22%2C%22lastName%22%3A%22Yan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valentina%22%2C%22lastName%22%3A%22Medici%22%7D%5D%2C%22abstractNote%22%3A%22The%20goal%20of%20this%20paper%20is%20to%20review%20current%20literature%20on%20nutritional%20ketosis%20within%20the%20context%20of%20weight%20management%20and%20metabolic%20syndrome%2C%20namely%2C%20insulin%20resistance%2C%20lipid%20profile%2C%20cardiovascular%20disease%20risk%2C%20and%20development%20of%20non-alcoholic%20fatty%20liver%20disease.%20We%20provide%20background%20on%20the%20mechanism%20of%20ketogenesis%20and%20describe%20nutritional%20ketosis.%22%2C%22date%22%3A%222018-09-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1007%5C%2Fs13668-018-0235-0%22%2C%22ISSN%22%3A%222161-3311%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs13668-018-0235-0%22%2C%22collections%22%3A%5B%226GWVSRHP%22%5D%2C%22dateModified%22%3A%222020-03-26T16%3A54%3A11Z%22%7D%7D%2C%7B%22key%22%3A%226NT2M8NW%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Volek%20and%20Sharman%22%2C%22parsedDate%22%3A%222004%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EVolek%20JS%2C%20Sharman%20MJ%20%282004%29%20Cardiovascular%20and%20Hormonal%20Aspects%20of%20Very-Low-Carbohydrate%20Ketogenic%20Diets.%20Obesity%20Research%2012%3A115S-123S.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Foby.2004.276%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Foby.2004.276%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Cardiovascular%20and%20Hormonal%20Aspects%20of%20Very-Low-Carbohydrate%20Ketogenic%20Diets%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeff%20S.%22%2C%22lastName%22%3A%22Volek%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthew%20J.%22%2C%22lastName%22%3A%22Sharman%22%7D%5D%2C%22abstractNote%22%3A%22VOLEK%2C%20JEFF%20S.%20AND%20MATTHEW%20J.%20SHARMAN.%20Cardiovascular%20and%20hormonal%20aspects%20of%20very-lowcarbohydrate%20ketogenic%20diets.%20Obes%20Res.%202004%3B12%3A%20115S%5Cu2013123S.%22%2C%22date%22%3A%2211%5C%2F2004%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1038%5C%2Foby.2004.276%22%2C%22ISSN%22%3A%2210717323%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1038%5C%2Foby.2004.276%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-19T17%3A34%3A29Z%22%7D%7D%2C%7B%22key%22%3A%223BUZRYYF%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kosinski%20and%20Jornayvaz%22%2C%22parsedDate%22%3A%222017-05-19%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EKosinski%20C%2C%20Jornayvaz%20F%20%282017%29%20Effects%20of%20Ketogenic%20Diets%20on%20Cardiovascular%20Risk%20Factors%3A%20Evidence%20from%20Animal%20and%20Human%20Studies.%20Nutrients%209%3A517.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fnu9050517%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fnu9050517%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Effects%20of%20Ketogenic%20Diets%20on%20Cardiovascular%20Risk%20Factors%3A%20Evidence%20from%20Animal%20and%20Human%20Studies%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Kosinski%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fran%5Cu00e7ois%22%2C%22lastName%22%3A%22Jornayvaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20treatment%20of%20obesity%20and%20cardiovascular%20diseases%20is%20one%20of%20the%20most%20dif%5Cufb01cult%20and%20important%20challenges%20nowadays.%20Weight%20loss%20is%20frequently%20offered%20as%20a%20therapy%20and%20is%20aimed%20at%20improving%20some%20of%20the%20components%20of%20the%20metabolic%20syndrome.%20Among%20various%20diets%2C%20ketogenic%20diets%2C%20which%20are%20very%20low%20in%20carbohydrates%20and%20usually%20high%20in%20fats%20and%5C%2For%20proteins%2C%20have%20gained%20in%20popularity.%20Results%20regarding%20the%20impact%20of%20such%20diets%20on%20cardiovascular%20risk%20factors%20are%20controversial%2C%20both%20in%20animals%20and%20humans%2C%20but%20some%20improvements%20notably%20in%20obesity%20and%20type%202%20diabetes%20have%20been%20described.%20Unfortunately%2C%20these%20effects%20seem%20to%20be%20limited%20in%20time.%20Moreover%2C%20these%20diets%20are%20not%20totally%20safe%20and%20can%20be%20associated%20with%20some%20adverse%20events.%20Notably%2C%20in%20rodents%2C%20development%20of%20nonalcoholic%20fatty%20liver%20disease%20%28NAFLD%29%20and%20insulin%20resistance%20have%20been%20described.%20The%20aim%20of%20this%20review%20is%20to%20discuss%20the%20role%20of%20ketogenic%20diets%20on%20different%20cardiovascular%20risk%20factors%20in%20both%20animals%20and%20humans%20based%20on%20available%20evidence.%22%2C%22date%22%3A%222017-05-19%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fnu9050517%22%2C%22ISSN%22%3A%222072-6643%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F2072-6643%5C%2F9%5C%2F5%5C%2F517%22%2C%22collections%22%3A%5B%229P7SYM5Z%22%5D%2C%22dateModified%22%3A%222020-03-19T17%3A25%3A20Z%22%7D%7D%2C%7B%22key%22%3A%22QYCRY2WY%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rusek%20et%20al.%22%2C%22parsedDate%22%3A%222019-08-09%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ERusek%20M%2C%20Pluta%20R%2C%20U%26%23x142%3Bamek-Kozio%26%23x142%3B%20M%2C%20Czuczwar%20SJ%20%282019%29%20Ketogenic%20Diet%20in%20Alzheimer%26%23x2019%3Bs%20Disease.%20IJMS%2020%3A3892.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijms20163892%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijms20163892%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Ketogenic%20Diet%20in%20Alzheimer%5Cu2019s%20Disease%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marta%22%2C%22lastName%22%3A%22Rusek%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ryszard%22%2C%22lastName%22%3A%22Pluta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marzena%22%2C%22lastName%22%3A%22U%5Cu0142amek-Kozio%5Cu0142%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stanis%5Cu0142aw%20J.%22%2C%22lastName%22%3A%22Czuczwar%22%7D%5D%2C%22abstractNote%22%3A%22At%20present%2C%20the%20prevalence%20of%20Alzheimer%5Cu2019s%20disease%2C%20a%20devastating%20neurodegenerative%20disorder%2C%20is%20increasing.%20Although%20the%20mechanism%20of%20the%20underlying%20pathology%20is%20not%20fully%20uncovered%2C%20in%20the%20last%20years%2C%20there%20has%20been%20signi%5Cufb01cant%20progress%20in%20its%20understanding.%20This%20includes%3A%20Progressive%20deposition%20of%20amyloid%20%5Cu03b2-peptides%20in%20amyloid%20plaques%20and%20hyperphosphorylated%20tau%20protein%20in%20intracellular%20as%20neuro%5Cufb01brillary%20tangles%3B%20neuronal%20loss%3B%20and%20impaired%20glucose%20metabolism.%20Due%20to%20a%20lack%20of%20e%5Cufb00ective%20prevention%20and%20treatment%20strategy%2C%20emerging%20evidence%20suggests%20that%20dietary%20and%20metabolic%20interventions%20could%20potentially%20target%20these%20issues.%20The%20ketogenic%20diet%20is%20a%20very%20high-fat%2C%20low-carbohydrate%20diet%2C%20which%20has%20a%20fasting-like%20e%5Cufb00ect%20bringing%20the%20body%20into%20a%20state%20of%20ketosis.%20The%20presence%20of%20ketone%20bodies%20has%20a%20neuroprotective%20impact%20on%20aging%20brain%20cells.%20Moreover%2C%20their%20production%20may%20enhance%20mitochondrial%20function%2C%20reduce%20the%20expression%20of%20in%5Cufb02ammatory%20and%20apoptotic%20mediators.%20Thus%2C%20it%20has%20gained%20interest%20as%20a%20potential%20therapy%20for%20neurodegenerative%20disorders%20like%20Alzheimer%5Cu2019s%20disease.%20This%20review%20aims%20to%20examine%20the%20role%20of%20the%20ketogenic%20diet%20in%20Alzheimer%5Cu2019s%20disease%20progression%20and%20to%20outline%20speci%5Cufb01c%20aspects%20of%20the%20nutritional%20pro%5Cufb01le%20providing%20a%20rationale%20for%20the%20implementation%20of%20dietary%20interventions%20as%20a%20therapeutic%20strategy%20for%20Alzheimer%5Cu2019s%20disease.%22%2C%22date%22%3A%222019-08-09%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fijms20163892%22%2C%22ISSN%22%3A%221422-0067%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F1422-0067%5C%2F20%5C%2F16%5C%2F3892%22%2C%22collections%22%3A%5B%22SPT2NJ53%22%5D%2C%22dateModified%22%3A%222020-03-19T17%3A15%3A25Z%22%7D%7D%2C%7B%22key%22%3A%22BNDJSCA5%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22W%5Cu0142odarek%22%2C%22parsedDate%22%3A%222019-01-15%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EW%26%23x142%3Bodarek%20D%20%282019%29%20Role%20of%20Ketogenic%20Diets%20in%20Neurodegenerative%20Diseases%20%28Alzheimer%26%23x2019%3Bs%20Disease%20and%20Parkinson%26%23x2019%3Bs%20Disease%29.%20Nutrients%2011%3A169.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fnu11010169%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fnu11010169%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Role%20of%20Ketogenic%20Diets%20in%20Neurodegenerative%20Diseases%20%28Alzheimer%5Cu2019s%20Disease%20and%20Parkinson%5Cu2019s%20Disease%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dariusz%22%2C%22lastName%22%3A%22W%5Cu0142odarek%22%7D%5D%2C%22abstractNote%22%3A%22The%20goal%20of%20this%20review%20was%20to%20assess%20the%20effectiveness%20of%20ketogenic%20diets%20on%20the%20therapy%20of%20neurodegenerative%20diseases.%20The%20ketogenic%20diet%20is%20a%20low-carbohydrate%20and%20fat-rich%20diet.%20Its%20implementation%20has%20a%20fasting-like%20effect%2C%20which%20brings%20the%20body%20into%20a%20state%20of%20ketosis.%20The%20ketogenic%20diet%20has%2C%20for%20almost%20100%20years%2C%20been%20used%20in%20the%20therapy%20of%20drug-resistant%20epilepsy%2C%20but%20current%20studies%20indicate%20possible%20neuroprotective%20effects.%20Thus%20far%2C%20only%20a%20few%20studies%20have%20evaluated%20the%20role%20of%20the%20ketogenic%20diet%20in%20the%20prevention%20of%20Parkinson%5Cu2019s%20disease%20%28PD%29%20and%20Alzheimer%5Cu2019s%20disease%20%28AD%29.%20Single%20studies%20with%20human%20participants%20have%20demonstrated%20a%20reduction%20of%20disease%20symptoms%20after%20application.%20The%20application%20of%20the%20ketogenic%20diet%20to%20elderly%20people%2C%20however%2C%20raises%20certain%20concerns.%20Persons%20with%20neurodegenerative%20diseases%20are%20at%20risk%20of%20malnutrition%2C%20while%20food%20intake%20reduction%20is%20associated%20with%20disease%20symptoms.%20In%20turn%2C%20the%20ketogenic%20diet%20leads%20to%20a%20reduced%20appetite%3B%20it%20is%20not%20attractive%20from%20an%20organoleptic%20point%20of%20view%2C%20and%20may%20be%20accompanied%20by%20side%20effects%20of%20the%20gastrointestinal%20system.%20All%20this%20may%20lead%20to%20further%20lowering%20of%20consumed%20food%20portions%20by%20elderly%20persons%20with%20neurodegenerative%20diseases%20and%2C%20in%20consequence%2C%20to%20further%20reduction%20in%20the%20supply%20of%20nutrients%20provided%20by%20the%20diet.%20Neither%20data%20on%20the%20long-term%20application%20of%20the%20ketogenic%20diet%20in%20patients%20with%20neurodegenerative%20disease%20or%20data%20on%20its%20effects%20on%20disease%20symptoms%20are%20available.%20Further%20research%20is%20needed%20to%20evaluate%20the%20suitability%20of%20the%20ketogenic%20diet%20in%20the%20therapy%20of%20AD-%20or%20PD-affected%20persons.%22%2C%22date%22%3A%222019-01-15%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fnu11010169%22%2C%22ISSN%22%3A%222072-6643%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F2072-6643%5C%2F11%5C%2F1%5C%2F169%22%2C%22collections%22%3A%5B%22SPT2NJ53%22%5D%2C%22dateModified%22%3A%222020-03-19T17%3A07%3A06Z%22%7D%7D%2C%7B%22key%22%3A%22Q6VKADKU%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ERole%20of%20Ketogenic%20Diets%20in%20Neurodegenerative%20Diseases%20%28Alzheimer%26%23x2019%3Bs%20Disease%20and%20Parkinson%26%23x2019%3Bs%20Disease%29.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpmc%5C%2Farticles%5C%2FPMC6356942%5C%2F%27%3Ehttps%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpmc%5C%2Farticles%5C%2FPMC6356942%5C%2F%3C%5C%2Fa%3E.%20Accessed%2019%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Role%20of%20Ketogenic%20Diets%20in%20Neurodegenerative%20Diseases%20%28Alzheimer%5Cu2019s%20Disease%20and%20Parkinson%5Cu2019s%20Disease%29%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpmc%5C%2Farticles%5C%2FPMC6356942%5C%2F%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222020-03-19T17%3A01%3A10Z%22%7D%7D%2C%7B%22key%22%3A%228YKAEID7%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Fine%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EFine%20E%20Targeting%20insulin%20inhibition%20as%20a%20metabolic%20therapy%20in%20advanced%20cancer%3A%20a%20pilot%20safety%20and%20feasibility%20dietary%20trial%20in%2010%20patients.%20-%20PubMed%20-%20NCBI.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F22840388%27%3Ehttps%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F22840388%3C%5C%2Fa%3E.%20Accessed%2019%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Targeting%20insulin%20inhibition%20as%20a%20metabolic%20therapy%20in%20advanced%20cancer%3A%20a%20pilot%20safety%20and%20feasibility%20dietary%20trial%20in%2010%20patients.%20-%20PubMed%20-%20NCBI%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E%22%2C%22lastName%22%3A%22Fine%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F22840388%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A57%3A25Z%22%7D%7D%2C%7B%22key%22%3A%229EYTNPQ2%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zhou%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EZhou%20W%20The%20calorically%20restricted%20ketogenic%20diet%2C%20an%20effective%20alternative%20therapy%20for%20malignant%20brain%20cancer.%20-%20PubMed%20-%20NCBI.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F17313687%27%3Ehttps%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F17313687%3C%5C%2Fa%3E.%20Accessed%2019%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22The%20calorically%20restricted%20ketogenic%20diet%2C%20an%20effective%20alternative%20therapy%20for%20malignant%20brain%20cancer.%20-%20PubMed%20-%20NCBI%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22W%22%2C%22lastName%22%3A%22Zhou%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F17313687%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A51%3A30Z%22%7D%7D%2C%7B%22key%22%3A%22Q7HKJ46T%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Klement%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EKlement%20Anti-Tumor%20Effects%20of%20Ketogenic%20Diets%20in%20Mice%3A%20A%20Meta-Analysis.%20-%20PubMed%20-%20NCBI.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F27159218%27%3Ehttps%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F27159218%3C%5C%2Fa%3E.%20Accessed%2019%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Anti-Tumor%20Effects%20of%20Ketogenic%20Diets%20in%20Mice%3A%20A%20Meta-Analysis.%20-%20PubMed%20-%20NCBI%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22%22%2C%22lastName%22%3A%22Klement%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpubmed%5C%2F27159218%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A45%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22LBX53LKX%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kentaro%20Nakamura%20et%20al.%22%2C%22parsedDate%22%3A%222018-02-14%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EKentaro%20Nakamura%2C%20Hidekazu%20Tonouchi%2C%20Akina%20Sasayama%2C%20Kinya%20Ashida%20%282018%29%20A%20Ketogenic%20Formula%20Prevents%20Tumor%20Progression%20and%20Cancer%20Cachexia%20by%20Attenuating%20Systemic%20Inflammation%20in%20Colon%2026%20Tumor-Bearing%20Mice.%20Nutrients%2010%3A206.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fnu10020206%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fnu10020206%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20Ketogenic%20Formula%20Prevents%20Tumor%20Progression%20and%20Cancer%20Cachexia%20by%20Attenuating%20Systemic%20Inflammation%20in%20Colon%2026%20Tumor-Bearing%20Mice%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Kentaro%20Nakamura%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Hidekazu%20Tonouchi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Akina%20Sasayama%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Kinya%20Ashida%22%7D%5D%2C%22abstractNote%22%3A%22Low-carbohydrate%2C%20high-fat%20diets%20%28ketogenic%20diets%29%20might%20prevent%20tumor%20progression%20and%20could%20be%20used%20as%20supportive%20therapy%3B%20however%2C%20few%20studies%20have%20addressed%20the%20effect%20of%20such%20diets%20on%20colorectal%20cancer.%20An%20infant%20formula%20with%20a%20ketogenic%20composition%20%28ketogenic%20formula%3B%20KF%29%20is%20used%20to%20treat%20patients%20with%20refractory%20epilepsy.%20We%20investigated%20the%20effect%20of%20KF%20on%20cancer%20and%20cancer%20cachexia%20in%20colon%20tumor-bearing%20mice.%20Mice%20were%20randomized%20into%20normal%20%28NR%29%2C%20tumor-bearing%20%28TB%29%2C%20and%20ketogenic%20formula%20%28KF%29%20groups.%20Colon%2026%20cells%20were%20inoculated%20subcutaneously%20into%20TB%20and%20KF%20mice.%20The%20NR%20and%20TB%20groups%20received%20a%20standard%20diet%2C%20and%20the%20KF%20mice%20received%20KF%20ad%20libitum.%20KF%20mice%20preserved%20their%20body%2C%20muscle%2C%20and%20carcass%20weights.%20Tumor%20weight%20and%20plasma%20IL-6%20levels%20were%20signi%5Cufb01cantly%20lower%20in%20KF%20mice%20than%20in%20TB%20mice.%20In%20the%20KF%20group%2C%20energy%20intake%20was%20signi%5Cufb01cantly%20higher%20than%20that%20in%20the%20other%20two%20groups.%20Blood%20ketone%20body%20concentrations%20in%20KF%20mice%20were%20signi%5Cufb01cantly%20elevated%2C%20and%20there%20was%20a%20signi%5Cufb01cant%20negative%20correlation%20between%20blood%20ketone%20body%20concentration%20and%20tumor%20weight.%20Therefore%2C%20KF%20may%20suppress%20the%20progression%20of%20cancer%20and%20the%20accompanying%20systemic%20in%5Cufb02ammation%20without%20adverse%20effects%20on%20weight%20gain%2C%20or%20muscle%20mass%2C%20which%20might%20help%20to%20prevent%20cancer%20cachexia.%22%2C%22date%22%3A%222018-02-14%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fnu10020206%22%2C%22ISSN%22%3A%222072-6643%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F2072-6643%5C%2F10%5C%2F2%5C%2F206%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A41%3A20Z%22%7D%7D%2C%7B%22key%22%3A%22CQQ6Y2IM%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lv%20et%20al.%22%2C%22parsedDate%22%3A%222014-12-11%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ELv%20M%2C%20Zhu%20X%2C%20Wang%20H%2C%20et%20al%20%282014%29%20Roles%20of%20Caloric%20Restriction%2C%20Ketogenic%20Diet%20and%20Intermittent%20Fasting%20during%20Initiation%2C%20Progression%20and%20Metastasis%20of%20Cancer%20in%20Animal%20Models%3A%20A%20Systematic%20Review%20and%20Meta-Analysis.%20PLoS%20ONE%209%3Ae115147.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0115147%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0115147%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Roles%20of%20Caloric%20Restriction%2C%20Ketogenic%20Diet%20and%20Intermittent%20Fasting%20during%20Initiation%2C%20Progression%20and%20Metastasis%20of%20Cancer%20in%20Animal%20Models%3A%20A%20Systematic%20Review%20and%20Meta-Analysis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mengmeng%22%2C%22lastName%22%3A%22Lv%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xingya%22%2C%22lastName%22%3A%22Zhu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hao%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Feng%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wenxian%22%2C%22lastName%22%3A%22Guan%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Guillermo%20L%5Cu00f3pez%22%2C%22lastName%22%3A%22Lluch%22%7D%5D%2C%22abstractNote%22%3A%22Background%3A%20The%20role%20of%20dietary%20restriction%20regimens%20such%20as%20caloric%20restriction%2C%20ketogenic%20diet%20and%20intermittent%20fasting%20in%20development%20of%20cancers%20has%20been%20detected%20via%20abundant%20preclinical%20experiments.%20However%2C%20the%20conclusions%20are%20controversial.%20We%20aim%20to%20review%20the%20relevant%20animal%20studies%20systematically%20and%20provide%20assistance%20for%20further%20clinical%20studies.%22%2C%22date%22%3A%222014-12-11%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0115147%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pone.0115147%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A34%3A06Z%22%7D%7D%2C%7B%22key%22%3A%22GM7HUCJD%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Poff%20et%20al.%22%2C%22parsedDate%22%3A%222013-06-05%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EPoff%20AM%2C%20Ari%20C%2C%20Seyfried%20TN%2C%20D%26%23x2019%3BAgostino%20DP%20%282013%29%20The%20Ketogenic%20Diet%20and%20Hyperbaric%20Oxygen%20Therapy%20Prolong%20Survival%20in%20Mice%20with%20Systemic%20Metastatic%20Cancer.%20PLoS%20ONE%208%3Ae65522.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0065522%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0065522%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20Ketogenic%20Diet%20and%20Hyperbaric%20Oxygen%20Therapy%20Prolong%20Survival%20in%20Mice%20with%20Systemic%20Metastatic%20Cancer%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Angela%20M.%22%2C%22lastName%22%3A%22Poff%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Csilla%22%2C%22lastName%22%3A%22Ari%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%20N.%22%2C%22lastName%22%3A%22Seyfried%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dominic%20P.%22%2C%22lastName%22%3A%22D%5Cu2019Agostino%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Chih-Hsin%22%2C%22lastName%22%3A%22Tang%22%7D%5D%2C%22abstractNote%22%3A%22Introduction%3A%20Abnormal%20cancer%20metabolism%20creates%20a%20glycolytic-dependency%20which%20can%20be%20exploited%20by%20lowering%20glucose%20availability%20to%20the%20tumor.%20The%20ketogenic%20diet%20%28KD%29%20is%20a%20low%20carbohydrate%2C%20high%20fat%20diet%20which%20decreases%20blood%20glucose%20and%20elevates%20blood%20ketones%20and%20has%20been%20shown%20to%20slow%20cancer%20progression%20in%20animals%20and%20humans.%20Abnormal%20tumor%20vasculature%20creates%20hypoxic%20pockets%20which%20promote%20cancer%20progression%20and%20further%20increase%20the%20glycolytic-dependency%20of%20cancers.%20Hyperbaric%20oxygen%20therapy%20%28HBO2T%29%20saturates%20tumors%20with%20oxygen%2C%20reversing%20the%20cancer%20promoting%20effects%20of%20tumor%20hypoxia.%20Since%20these%20non-toxic%20therapies%20exploit%20overlapping%20metabolic%20deficiencies%20of%20cancer%2C%20we%20tested%20their%20combined%20effects%20on%20cancer%20progression%20in%20a%20natural%20model%20of%20metastatic%20disease.%5CnMethods%3A%20We%20used%20the%20firefly%20luciferase-tagged%20VM-M3%20mouse%20model%20of%20metastatic%20cancer%20to%20compare%20tumor%20progression%20and%20survival%20in%20mice%20fed%20standard%20or%20KD%20ad%20libitum%20with%20or%20without%20HBO2T%20%282.5%20ATM%20absolute%2C%2090%20min%2C%203x%5C%2Fweek%29.%20Tumor%20growth%20was%20monitored%20by%20in%20vivo%20bioluminescent%20imaging.%5CnResults%3A%20KD%20alone%20significantly%20decreased%20blood%20glucose%2C%20slowed%20tumor%20growth%2C%20and%20increased%20mean%20survival%20time%20by%2056.7%25%20in%20mice%20with%20systemic%20metastatic%20cancer.%20While%20HBO2T%20alone%20did%20not%20influence%20cancer%20progression%2C%20combining%20the%20KD%20with%20HBO2T%20elicited%20a%20significant%20decrease%20in%20blood%20glucose%2C%20tumor%20growth%20rate%2C%20and%2077.9%25%20increase%20in%20mean%20survival%20time%20compared%20to%20controls.%5CnConclusions%3A%20KD%20and%20HBO2T%20produce%20significant%20anti-cancer%20effects%20when%20combined%20in%20a%20natural%20model%20of%20systemic%20metastatic%20cancer.%20Our%20evidence%20suggests%20that%20these%20therapies%20should%20be%20further%20investigated%20as%20potential%20non-toxic%20treatments%20or%20adjuvant%20therapies%20to%20standard%20care%20for%20patients%20with%20systemic%20metastatic%20disease.%22%2C%22date%22%3A%222013-6-5%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0065522%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pone.0065522%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A29%3A05Z%22%7D%7D%2C%7B%22key%22%3A%22SWT7IIDA%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Poff%20et%20al.%22%2C%22parsedDate%22%3A%222019%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EPoff%20A%2C%20Koutnik%20AP%2C%20Egan%20KM%2C%20et%20al%20%282019%29%20Targeting%20the%20Warburg%20effect%20for%20cancer%20treatment%3A%20Ketogenic%20diets%20for%20management%20of%20glioma.%20Seminars%20in%20Cancer%20Biology%2056%3A135%26%23x2013%3B148.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.semcancer.2017.12.011%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.semcancer.2017.12.011%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Targeting%20the%20Warburg%20effect%20for%20cancer%20treatment%3A%20Ketogenic%20diets%20for%20management%20of%20glioma%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Angela%22%2C%22lastName%22%3A%22Poff%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrew%20P.%22%2C%22lastName%22%3A%22Koutnik%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kathleen%20M.%22%2C%22lastName%22%3A%22Egan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Solmaz%22%2C%22lastName%22%3A%22Sahebjam%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dominic%22%2C%22lastName%22%3A%22D%5Cu2019Agostino%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nagi%20B.%22%2C%22lastName%22%3A%22Kumar%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2206%5C%2F2019%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.semcancer.2017.12.011%22%2C%22ISSN%22%3A%221044579X%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1044579X17301244%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A11%3A01Z%22%7D%7D%2C%7B%22key%22%3A%22GWS8CT5H%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Sremanakova%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ESremanakova%20A%20systematic%20review%20of%20the%20use%20of%20ketogenic%20diets%20in%20adult%20patients%20with%20cancer%20-%20Sremanakova%20-%202018%20-%20Journal%20of%20Human%20Nutrition%20and%20Dietetics%20-%20Wiley%20Online%20Library.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fonlinelibrary.wiley.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1111%5C%2Fjhn.12587%27%3Ehttps%3A%5C%2F%5C%2Fonlinelibrary.wiley.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1111%5C%2Fjhn.12587%3C%5C%2Fa%3E.%20Accessed%2019%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22A%20systematic%20review%20of%20the%20use%20of%20ketogenic%20diets%20in%20adult%20patients%20with%20cancer%20-%20Sremanakova%20-%202018%20-%20Journal%20of%20Human%20Nutrition%20and%20Dietetics%20-%20Wiley%20Online%20Library%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22%22%2C%22lastName%22%3A%22Sremanakova%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fonlinelibrary.wiley.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1111%5C%2Fjhn.12587%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A03%3A12Z%22%7D%7D%2C%7B%22key%22%3A%224RDMGN6M%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bettum%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EBettum%20IJ%2C%20Gorad%20SS%2C%20Barkovskaya%20A%2C%20et%20al%20%282015%29%20Metabolic%20reprogramming%20supports%20the%20invasive%20phenotype%20in%20malignant%20melanoma.%20Cancer%20Letters%20366%3A71%26%23x2013%3B83.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.canlet.2015.06.006%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.canlet.2015.06.006%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Metabolic%20reprogramming%20supports%20the%20invasive%20phenotype%20in%20malignant%20melanoma%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ingrid%20J.%22%2C%22lastName%22%3A%22Bettum%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Saurabh%20S.%22%2C%22lastName%22%3A%22Gorad%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Barkovskaya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Solveig%22%2C%22lastName%22%3A%22Pettersen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Siver%20A.%22%2C%22lastName%22%3A%22Moestue%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kotryna%22%2C%22lastName%22%3A%22Vasiliauskaite%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ellen%22%2C%22lastName%22%3A%22Tenstad%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tove%22%2C%22lastName%22%3A%22%5Cu00d8yjord%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22%5Cu00d8ystein%22%2C%22lastName%22%3A%22Risa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vigdis%22%2C%22lastName%22%3A%22Nygaard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gunhild%20M.%22%2C%22lastName%22%3A%22M%5Cu00e6landsmo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lina%22%2C%22lastName%22%3A%22Prasmickaite%22%7D%5D%2C%22abstractNote%22%3A%22Invasiveness%20is%20a%20hallmark%20of%20aggressive%20cancer%20like%20malignant%20melanoma%2C%20and%20factors%20involved%20in%20acquisition%20or%20maintenance%20of%20an%20invasive%20phenotype%20are%20attractive%20targets%20for%20therapy.%20We%20investigated%20melanoma%20phenotype%20modulation%20induced%20by%20the%20metastasis-promoting%20microenvironmental%20protein%20S100A4%2C%20focusing%20on%20the%20relationship%20between%20enhanced%20cellular%20motility%2C%20dedifferentiation%20and%20metabolic%20changes.%20In%20poorly%20motile%2C%20well-differentiated%20Melmet%205%20cells%2C%20S100A4%20stimulated%20migration%2C%20invasion%20and%20simultaneously%20down-regulated%20differentiation%20genes%20and%20modulated%20expression%20of%20metabolism%20genes.%20Metabolic%20studies%20con%5Cufb01rmed%20suppressed%20mitochondrial%20respiration%20and%20activated%20glycolytic%20%5Cufb02ux%20in%20the%20S100A4%20stimulated%20cells%2C%20indicating%20a%20metabolic%20switch%20toward%20aerobic%20glycolysis%2C%20known%20as%20the%20Warburg%20effect.%20Reversal%20of%20the%20glycolytic%20switch%20by%20dichloracetate%20induced%20apoptosis%20and%20reduced%20cell%20growth%2C%20particularly%20in%20the%20S100A4%20stimulated%20cells.%20This%20implies%20that%20cells%20with%20stimulated%20invasiveness%20get%20survival%20bene%5Cufb01t%20from%20the%20glycolytic%20switch%20and%2C%20therefore%2C%20become%20more%20vulnerable%20to%20glycolysis%20inhibition.%20In%20conclusion%2C%20our%20data%20indicate%20that%20transition%20to%20the%20invasive%20phenotype%20in%20melanoma%20involves%20dedifferentiation%20and%20metabolic%20reprogramming%20from%20mitochondrial%20oxidation%20to%20glycolysis%2C%20which%20facilitates%20survival%20of%20the%20invasive%20cancer%20cells.%20Therapeutic%20strategies%20targeting%20the%20metabolic%20reprogramming%20may%20therefore%20be%20effective%20against%20the%20invasive%20phenotype.%22%2C%22date%22%3A%2209%5C%2F2015%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.canlet.2015.06.006%22%2C%22ISSN%22%3A%2203043835%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS0304383515003985%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A01%3A54Z%22%7D%7D%2C%7B%22key%22%3A%22D9QF3U55%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Hanahan%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EHanahan%20Hallmarks%20of%20Cancer%3A%20The%20Next%20Generation%3A%20Cell.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.cell.com%5C%2Fcell%5C%2Ffulltext%5C%2FS0092-8674%2811%2900127-9%3F_returnURL%3Dhttps%253A%252F%252Flinkinghub.elsevier.com%252Fretrieve%252Fpii%252FS0092867411001279%253Fshowall%253Dtrue%27%3Ehttps%3A%5C%2F%5C%2Fwww.cell.com%5C%2Fcell%5C%2Ffulltext%5C%2FS0092-8674%2811%2900127-9%3F_returnURL%3Dhttps%253A%252F%252Flinkinghub.elsevier.com%252Fretrieve%252Fpii%252FS0092867411001279%253Fshowall%253Dtrue%3C%5C%2Fa%3E.%20Accessed%2019%20Mar%202020%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Hallmarks%20of%20Cancer%3A%20The%20Next%20Generation%3A%20Cell%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22%22%2C%22lastName%22%3A%22Hanahan%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.cell.com%5C%2Fcell%5C%2Ffulltext%5C%2FS0092-8674%2811%2900127-9%3F_returnURL%3Dhttps%253A%252F%252Flinkinghub.elsevier.com%252Fretrieve%252Fpii%252FS0092867411001279%253Fshowall%253Dtrue%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T16%3A00%3A21Z%22%7D%7D%2C%7B%22key%22%3A%22BT3F4UR4%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Warburg%22%2C%22parsedDate%22%3A%221956-02-24%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EWarburg%20O%20%281956%29%20On%20the%20Origin%20of%20Cancer%20Cells.%20Science%20123%3A309%26%23x2013%3B314.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1126%5C%2Fscience.123.3191.309%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1126%5C%2Fscience.123.3191.309%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22On%20the%20Origin%20of%20Cancer%20Cells%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22O.%22%2C%22lastName%22%3A%22Warburg%22%7D%5D%2C%22abstractNote%22%3A%22Cancer%20cells%20originate%20from%20normal%20body%20cells%20in%20two%20phases.%20The%20first%20phase%20is%20the%20irreversible%20injuring%20of%20respiration.%20Just%20as%20there%20are%20many%20remote%20causes%20of%20plague-heat%2C%20insects%2C%20rats-but%20only%20one%20common%20cause%2C%20the%20plague%20bacillus%2C%20there%20are%20a%20great%20many%20remote%20causes%20of%20cancer-tar%2C%20rays%2C%20arsenic%2C%20pressure%2C%20urethane-but%20there%20is%20only%20one%20common%20cause%20into%20which%20all%20other%20causes%20of%20cancer%20merge%2C%20the%20irreversible%20injuring%20of%20respiration.%22%2C%22date%22%3A%221956-02-24%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1126%5C%2Fscience.123.3191.309%22%2C%22ISSN%22%3A%220036-8075%2C%201095-9203%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencemag.org%5C%2Flookup%5C%2Fdoi%5C%2F10.1126%5C%2Fscience.123.3191.309%22%2C%22collections%22%3A%5B%225NYZ8VT6%22%5D%2C%22dateModified%22%3A%222020-03-19T15%3A53%3A30Z%22%7D%7D%2C%7B%22key%22%3A%22SIDRIKSV%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Van%20Wymelbeke%20et%20al.%22%2C%22parsedDate%22%3A%221998-08%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EVan%20Wymelbeke%20V%2C%20Himaya%20A%2C%20Louis-Sylvestre%20J%2C%20Fantino%20M%20%281998%29%20Influence%20of%20medium-chain%20and%20long-chain%20triacylglycerols%20on%20the%20control%20of%20food%20intake%20in%20men.%20Am%20J%20Clin%20Nutr%2068%3A226%26%23x2013%3B234.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fajcn%5C%2F68.2.226%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fajcn%5C%2F68.2.226%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Influence%20of%20medium-chain%20and%20long-chain%20triacylglycerols%20on%20the%20control%20of%20food%20intake%20in%20men%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22V.%22%2C%22lastName%22%3A%22Van%20Wymelbeke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Himaya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Louis-Sylvestre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Fantino%22%7D%5D%2C%22abstractNote%22%3A%22Hunger%20may%20be%20delayed%20and%20food%20intake%20reduced%20under%20metabolic%20conditions%20that%20spare%20carbohydrate%20oxidation%2C%20especially%20during%20oxidation%20of%20medium-chain%20triacylglycerols%20%28MCTs%29%20or%20monounsaturated%20triacylglycerols.%20In%2012%20healthy%2C%20adult%2C%20male%20volunteers%20isolated%20and%20deprived%20of%20any%20time%20cues%2C%20we%20compared%20the%20effects%20of%204%20high-carbohydrate%20breakfasts%20%281670%20kJ%29%20supplemented%20either%20with%20a%20fat%20substitute%20%28Sub%3B%2070%20kJ%29%20or%20with%201460%20kJ%20fat%20as%20monounsaturated%20long-chain%20triacylglycerols%20%28LCT-U%29%2C%20saturated%20long-chain%20triacylglycerols%20%28LCT-S%29%2C%20or%20MCTs.%20In%20the%20first%20session%20we%20investigated%20the%20effects%20of%20these%20breakfasts%20on%20the%20following%20food%20intake%20variables%3A%20hunger%20ratings%20at%20repeated%20intervals%2C%20the%20time%20until%20the%20spontaneous%20request%20for%20the%20next%202%20free-choice%20meals%2C%20and%20the%20amount%20of%20food%20consumed.%20In%20a%20second%20session%20with%20fixed%20lunches%2C%20we%20studied%20the%20effects%20of%20the%20same%20breakfasts%20on%20plasma%20glucose%2C%20insulin%2C%20triacylglycerol%2C%20fatty%20acid%2C%20and%20beta-hydroxybutyrate%20concentrations.%20The%20addition%20of%20any%20of%20the%20fats%20to%20the%20high-carbohydrate%20breakfasts%20did%20not%20alter%20hunger%20ratings%2C%20but%20significantly%20delayed%20the%20request%20for%20lunch%20compared%20with%20the%20low-fat%20breakfast.%20The%20free-choice%20lunch%20eaten%20after%20the%20MCT%20breakfast%20was%20also%20significantly%20smaller.%20Blood%20glucose%20and%20insulin%20concentrations%20were%20lower%20after%20the%203%20fat%20breakfasts%2C%20followed%20by%20larger%20increases%20in%20glucose%20and%20enhanced%20insulin%20responses%2030%20min%20after%20the%20lunch.%20No%20differences%20were%20observed%20between%20the%20LCT-U%20and%20LCT-S%20conditions.%20We%20conclude%20that%20MCTs%20decreased%20food%20intake%20by%20a%20postabsorptive%20mechanism%2C%20although%20the%20exact%20effect%20of%20these%20lipids%20on%20carbohydrate%20oxidation%20will%20require%20further%20studies%20involving%20nutrient%20balance%20measurements.%22%2C%22date%22%3A%22Aug%201998%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1093%5C%2Fajcn%5C%2F68.2.226%22%2C%22ISSN%22%3A%220002-9165%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22HJKVZCPI%22%5D%2C%22dateModified%22%3A%222020-03-17T04%3A38%3A54Z%22%7D%7D%2C%7B%22key%22%3A%222KSJLCV8%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Hellman%20et%20al.%22%2C%22parsedDate%22%3A%221969-11-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EHellman%20DE%2C%20Senior%20B%2C%20Goodman%20HM%20%281969%29%20Anti-lipolytic%20effects%20of%20%26%23x3B2%3B-hydroxybutyrate.%20Metabolism%20-%20Clinical%20and%20Experimental%2018%3A906%26%23x2013%3B915.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2F0026-0495%2869%2990031-6%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2F0026-0495%2869%2990031-6%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Anti-lipolytic%20effects%20of%20%5Cu03b2-hydroxybutyrate%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dorothea%20E.%22%2C%22lastName%22%3A%22Hellman%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Boris%22%2C%22lastName%22%3A%22Senior%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H.%20Maurice%22%2C%22lastName%22%3A%22Goodman%22%7D%5D%2C%22abstractNote%22%3A%22%3Ch2%3EAbstract%3C%5C%2Fh2%3E%3Cp%3EThe%20addition%20of%20%5Cu03b2-hydroxybutyrate%20to%20epididymal%20fat%20incubated%20in%20vitro%20reduced%20the%20production%20of%20glycerol%20and%20free%20fatty%20acids.%20Inhibition%20of%20lipolysis%20was%20maximal%20%28%5Cu223c%2030%25%29%20when%20physiologic%20concentrations%20of%20%5Cu03b2-hydroxybutyrate%20%280.1%20mg.%5C%2Fml.%29%20were%20added%20to%20tissues%20obtained%20from%20fasting%20rats.%20At%20a%20concentration%20of%2010%20mg.%5C%2Fml.%2C%20%5Cu03b2-hydroxybutyrate%20also%20abolished%20lipolysis%20in%20response%20to%200.05%20%5Cu03bcg.%5C%2Fml.%20epinephrine%20and%20severely%20reduced%20the%20lipolytic%20effects%20of%20ACTH%20%281%20%5Cu03bcg.%5C%2Fml.%29%2C%20TSH%20%2825%20mU%5C%2Fml.%29%20and%20glucagon%20%2810%20%5Cu03bcg.%5C%2Fml.%29.%20Increasing%20the%20concentration%20of%20epinephrine%20produced%20a%20progressive%20but%20significantly%20lesser%20lipolytic%20response%20in%20%5Cu03b2-hydroxybutyrate%20treated%20%2810%20mg.%5C%2Fml.%29%20than%20in%20control%20tissues.%20%5Cu03b2-hydroxybutyrate%2C%2010%20mg.%5C%2Fml.%2C%20abolished%20the%20lipolytic%20response%20to%20theophylline%20%280.54%20mg.%5C%2Fml.%29%2C%20but%20did%20not%20inhibit%20the%20lipolytic%20response%20to%20exogenous%20cyclic%20adenosine%203%5Cu2032%2C5%5Cu2032-monophosphate%20%28CAMP%29%20in%20the%20presence%20of%20theophylline.%20These%20results%20are%20consistent%20with%20the%20concept%20that%20%5Cu03b2-hydroxbutyrate%20may%20modulate%20the%20production%20of%20fatty%20acids%20during%20fasting%20and%20suggest%20that%20the%20antagonism%20of%20epinephrine-induced%20lipolysis%20may%20result%20from%20inhibition%20of%20CAMP%20formation%20in%20adipose%20tissue.%3C%5C%2Fp%3E%22%2C%22date%22%3A%221969%5C%2F11%5C%2F01%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2F0026-0495%2869%2990031-6%22%2C%22ISSN%22%3A%220026-0495%2C%201532-8600%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.metabolismjournal.com%5C%2Farticle%5C%2F0026-0495%2869%2990031-6%5C%2Fabstract%22%2C%22collections%22%3A%5B%22TPPQF3UK%22%5D%2C%22dateModified%22%3A%222020-03-16T09%3A51%3A18Z%22%7D%7D%2C%7B%22key%22%3A%225JRYL8UX%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Newport%20et%20al.%22%2C%22parsedDate%22%3A%222015-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ENewport%20MT%2C%20VanItallie%20TB%2C%20Kashiwaya%20Y%2C%20et%20al%20%282015%29%20A%20new%20way%20to%20produce%20hyperketonemia%3A%20use%20of%20ketone%20ester%20in%20a%20case%20of%20Alzheimer%26%23x2019%3Bs.%20Alzheimers%20Dement%2011%3A99%26%23x2013%3B103.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jalz.2014.01.006%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jalz.2014.01.006%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20new%20way%20to%20produce%20hyperketonemia%3A%20use%20of%20ketone%20ester%20in%20a%20case%20of%20Alzheimer%5Cu2019s%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mary%20T.%22%2C%22lastName%22%3A%22Newport%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Theodore%20B.%22%2C%22lastName%22%3A%22VanItallie%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yoshihiro%22%2C%22lastName%22%3A%22Kashiwaya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%20Todd%22%2C%22lastName%22%3A%22King%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%20L.%22%2C%22lastName%22%3A%22Veech%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-1%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.jalz.2014.01.006%22%2C%22ISSN%22%3A%221552-5260%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpmc%5C%2Farticles%5C%2FPMC4300286%5C%2F%22%2C%22collections%22%3A%5B%22TPPQF3UK%22%5D%2C%22dateModified%22%3A%222020-03-16T01%3A51%3A50Z%22%7D%7D%2C%7B%22key%22%3A%22QU6JIB96%22%2C%22library%22%3A%7B%22id%22%3A5748374%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Cavaleri%20and%20Bashar%22%2C%22parsedDate%22%3A%222018-04-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%20%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ECavaleri%20F%2C%20Bashar%20E%20%282018%29%20Potential%20Synergies%20of%20%26%23x3B2%3B-Hydroxybutyrate%20and%20Butyrate%20on%20the%20Modulation%20of%20Metabolism%2C%20Inflammation%2C%20Cognition%2C%20and%20General%20Health.%20J%20Nutr%20Metab%202018%3A.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1155%5C%2F2018%5C%2F7195760%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1155%5C%2F2018%5C%2F7195760%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Potential%20Synergies%20of%20%5Cu03b2-Hydroxybutyrate%20and%20Butyrate%20on%20the%20Modulation%20of%20Metabolism%2C%20Inflammation%2C%20Cognition%2C%20and%20General%20Health%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Franco%22%2C%22lastName%22%3A%22Cavaleri%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emran%22%2C%22lastName%22%3A%22Bashar%22%7D%5D%2C%22abstractNote%22%3A%22The%20low-carbohydrate%20high-fat%20diet%20%28LCHFD%29%2C%20also%20known%20as%20the%20ketogenic%20diet%2C%20has%20cycled%20in%20and%20out%20of%20popularity%20for%20decades%20as%20a%20therapeutic%20program%20to%20treat%20metabolic%20syndrome%2C%20weight%20mismanagement%2C%20and%20drug-resistant%20disorders%20as%20complex%20as%20epilepsy%2C%20cancer%2C%20dementia%2C%20and%20depression.%20Despite%20the%20benefits%20of%20this%20diet%2C%20health%20care%20professionals%20still%20question%20its%20safety%20due%20to%20the%20elevated%20serum%20ketones%20it%20induces%20and%20the%20limited%20dietary%20fiber.%20To%20compound%20the%20controversy%2C%20patient%20compliance%20with%20the%20program%20is%20poor%20due%20to%20the%20restrictive%20nature%20of%20the%20diet%20and%20symptoms%20related%20to%20energy%20deficit%20and%20gastrointestinal%20adversity%20during%20the%20introductory%20and%20energy%20substrate%20transition%20phase%20of%20the%20diet.%20The%20studies%20presented%20here%20demonstrate%20safety%20and%20efficacy%20of%20the%20diet%20including%20the%20scientific%20support%20and%20rationale%20for%20the%20administration%20of%20exogenous%20ketone%20bodies%20and%20ketone%20sources%20as%20a%20complement%20to%20the%20restrictive%20dietary%20protocol%20or%20as%20an%20alternative%20to%20the%20diet.%20This%20review%20also%20highlights%20the%20synergy%20provided%20by%20exogenous%20ketone%2C%20%5Cu03b2-hydroxybutyrate%20%28BHB%29%2C%20accompanied%20by%20the%20short%20chain%20fatty%20acid%2C%20butyrate%20%28BA%29%20in%20the%20context%20of%20cellular%20and%20physiological%20outcomes.%20More%20work%20is%20needed%20to%20unveil%20the%20molecular%20mechanisms%20by%20which%20this%20program%20provides%20health%20benefits.%22%2C%22date%22%3A%222018-4-1%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1155%5C%2F2018%5C%2F7195760%22%2C%22ISSN%22%3A%222090-0724%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpmc%5C%2Farticles%5C%2FPMC5902005%5C%2F%22%2C%22collections%22%3A%5B%22TPPQF3UK%22%5D%2C%22dateModified%22%3A%222020-03-16T01%3A37%3A17Z%22%7D%7D%5D%7D
1.
Dietze G, Wicklmayr M, Grunst J, Mehnert H (1976) [The anti-ketogenic effect of fructose]. Verh Dtsch Ges Inn Med 82 Pt 1:767–769
1.
Lee BM, Wolever TM (1998) Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: comparison with white bread. Eur J Clin Nutr 52:924–928.
https://doi.org/10.1038/sj.ejcn.1600666
1.
Pawlosky RJ, Kashiwaya Y, King MT, Veech RL (2020) A Dietary Ketone Ester Normalizes Abnormal Behavior in a Mouse Model of Alzheimer’s Disease. IJMS 21:1044.
https://doi.org/10.3390/ijms21031044
1.
Poulain M, Pes GM, Grasland C, et al (2004) Identification of a geographic area characterized by extreme longevity in the Sardinia island: the AKEA study. Experimental Gerontology 39:1423–1429.
https://doi.org/10.1016/j.exger.2004.06.016
1.
Esposito K, Chiodini P, Colao A, et al (2012) Metabolic Syndrome and Risk of Cancer: A systematic review and meta-analysis. Diabetes Care 35:2402–2411.
https://doi.org/10.2337/dc12-0336
1.
Seeman TE, Crimmins E, Huang M-H, et al (2004) Cumulative biological risk and socio-economic differences in mortality: MacArthur Studies of Successful Aging. Social Science & Medicine 58:1985–1997.
https://doi.org/10.1016/S0277-9536(03)00402-7
1.
Pinquart M, Sörensen S (2000) Influences of socioeconomic status, social network, and competence on subjective well-being in later life: A meta-analysis. Psychology and Aging 15:187–224.
https://doi.org/10.1037/0882-7974.15.2.187
1.
Lemaître J-F, Ronget V, Tidière M, et al (2020) Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc Natl Acad Sci USA.
https://doi.org/10.1073/pnas.1911999117
1.
Madsen EL, Rissanen A, Bruun JM, et al (2008) Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. European Journal of Endocrinology 158:179–187.
https://doi.org/10.1530/EJE-07-0721
1.
Nguyen NT, Nguyen X-MT, Wooldridge JB, et al (2010) Association of obesity with risk of coronary heart disease: findings from the National Health and Nutrition Examination Survey, 1999–2006. Surgery for Obesity and Related Diseases 6:465–469.
https://doi.org/10.1016/j.soard.2010.02.038
1.
Partsalaki I, Karvela A, Spiliotis BE (2012) Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J Pediatr Endocrinol Metab 25:697–704.
https://doi.org/10.1515/jpem-2012-0131
1.
Kosinski C, Jornayvaz F (2017) Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 9:517.
https://doi.org/10.3390/nu9050517
1.
Włodarek D (2019) Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 11:169.
https://doi.org/10.3390/nu11010169
1.
Fine E Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. - PubMed - NCBI.
https://www.ncbi.nlm.nih.gov/pubmed/22840388. Accessed 19 Mar 2020
1.
Kentaro Nakamura, Hidekazu Tonouchi, Akina Sasayama, Kinya Ashida (2018) A Ketogenic Formula Prevents Tumor Progression and Cancer Cachexia by Attenuating Systemic Inflammation in Colon 26 Tumor-Bearing Mice. Nutrients 10:206.
https://doi.org/10.3390/nu10020206
1.
Lv M, Zhu X, Wang H, et al (2014) Roles of Caloric Restriction, Ketogenic Diet and Intermittent Fasting during Initiation, Progression and Metastasis of Cancer in Animal Models: A Systematic Review and Meta-Analysis. PLoS ONE 9:e115147.
https://doi.org/10.1371/journal.pone.0115147
1.
Poff AM, Ari C, Seyfried TN, D’Agostino DP (2013) The Ketogenic Diet and Hyperbaric Oxygen Therapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLoS ONE 8:e65522.
https://doi.org/10.1371/journal.pone.0065522
1.
Van Wymelbeke V, Himaya A, Louis-Sylvestre J, Fantino M (1998) Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr 68:226–234.
https://doi.org/10.1093/ajcn/68.2.226
1.
Cavaleri F, Bashar E (2018) Potential Synergies of β-Hydroxybutyrate and Butyrate on the Modulation of Metabolism, Inflammation, Cognition, and General Health. J Nutr Metab 2018:.
https://doi.org/10.1155/2018/7195760



